- ▶
- Heaters/Source
- ▶
- Agilent Heaters and SensorsMass Spectrometry, Scientific Supplies & ManufacturingScientific Instrument Services 5973 Source Heater Tamper Resistant Allen Wrench 5973/5975 Quad Sensor 5985 Source Heater Assembly Agilent Interface Heater Assembly 5971 Interface Heater

- ▶
- Reference Material on InstrumentationArticle - A High Temperature Direct Probe for a Mass Spectrometer Design of a Direct Exposure Probe and Controller for use ona Hewlett-Packard 5989 Mass Spectrometer SIS AP1000 AutoProbe™ SIS AP2000 AutoProbe™ - Description of System HPP7: Direct Probe Electronics Console HPP7: Direct Probe for the Agilent (HP) 5973/5975 MSD HPP7: HP Direct Probe Application Notes HPP7: Installation Directions for the Direct Probe HPP7: Side Cover for the HP 5973 MSD HPP7: Support HPP7: Probe Inlet System for the Agilent (HP) 5973 and 5975 MSD with Automatic Indexed Stops HPP7: Theory of Operation of the Direct Probe and Probe Inlet System Direct Thermal Extraction Thermal Desorption Application Notes Environmental Thermal Desorption Application Notes Food Science Thermal Desorption Application Notes Forensic Thermal Desorption Application Notes GC Cryo-Trap Application Notes Headspace Application Notes Purge & Trap Thermal Desorption Application Notes Theory of Operation of the AutoDesorb® System AutoDesorb Notes for SIS Dealers Adsorbent Resin Application Notes Installation of the Short Path Thermal Desorption System on Agilent (HP) and Other GCs Installation of the Short Path Thermal Desorption System on a Varian 3400 GC AutoDesorb® System Development Team Thermal Desorption Applications and Reference Materials Installation of the Short Path Thermal Desorption System - TD5 Part I - Design & Operation of the Short Path ThermalDesorption System Installation Instructions for the Model 951 GC Cryo-Trap on the HP 5890 Series GC Installation Instructions for the Model 961 GC Cryo-Trap on the HP 5890 Series GC Operation of the Model 951/961 GC Cryo-Trap SIS GC Cryo Traps - Theory of Operation NIST/EPA/NIH Mass Spectral Enhancements - 1998 version (NIST98) SIMION 3D Ion Optics Class Mass Spectrometer Source Cleaning Methods MS Tip: Mass Spectrometer Source Cleaning Procedures Mass Spec Source Cleaning Procedures Micro-Mesh® Abrasive Sheets Research Papers Using New Era Syringe Pump Systems EI Positive Ion Spectra for Perfluorokerosene (PFK) Cap Liner Information How do I convert between fluid oz and milliliters? Which bottle material should I choose? Which bottle mouth should I choose? The Bottle Selection Guide CGA Connections for Gas Tanks Chemical Reaction Interface Mass Spectrometry (CRIMS)

- TD
- ▶
- AccessoriesTD Supply Kit Desorption Tubes Adsorbent Resins Desorption Tube Needles Desorption Tube Seals Desorption System Fittings GC Cryo-Trap Extraction Cell TD Sample Loader Prepacked, Conditioned Desorption Tubes Desorption Tube Packing Accessories Stainless Steel Purge Heads Injection Port Liners Tenax TA Poster TD Application Notes Customer Service

- LiteratureApplication Notes Adsorbent Resins Guide Mass Spec Tips SDS Sheets FAQ MS Calibration Compound Spectra Manuals MS Links/Labs/ Organizations MS Online Tools Flyers on Products/Services Scientific Supplies Catalog About Us NextAdvance Bullet Blender® Homogenizer Protocols Micro-Mesh® Literature Instrumentation Literature Agilent GC/MS Literature SIS News / E-Mail Newsletter NIST MS Database - Update Notifications

- ▶
- Thermal Desorption Applications and Reference MaterialsDirect Thermal Extraction Headspace Environmental Food Science Applications Pharmaceuticals Forensic Note 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 57: Aroma Profiles of Lavandula species Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS

- Application NotesNote 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 96: Reducing Warping in Mass Spectrometer Filaments, with SISAlloy® Yttria/Rhenium Filaments Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 92: Yttria Coated Mass Spectrometer Filaments Note 91: AutoProbe DEP Probe Tip Temperatures Note 90: An Automated MS Direct Probe for use in an Open Access Environment Note 89: Quantitation of Organics via a Mass Spectrometer Automated Direct Probe Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 87: Design and Development of an Automated Direct Probe for a Mass Spectrometer Note 86: Simulation of a Unique Cylindrical Quadrupole Mass Analyzer Using SIMION 7.0. Note 85: Replacing an Electron Multiplier in the Agilent (HP) 5973 MSD Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 81: Rapid Bacterial Chemotaxonomy By DirectProbe/MSD Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 78: A New Solution to Eliminate MS Down-Time With No-Tool-Changing of Analytical GC Columns Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 76: Determination of the Sensitivity of a CRIMS System Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 72: 1998 Version of the NIST/EPA/NIH Mass Spectral Library, NIST98 Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 70: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part II Note 69: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part 1 Note 68: Use of a PC Plug-In UV-Vis Spectrometer To Monitor the Plasma Conditions In GC-CRIMS Note 67: Using Chemical Reaction Interface Mass Spectrometry (CRIMS) To Monitor Bacterial Transport In In Situ Bioremediation Note 66: Probe Tip Design For the Optimization of Direct Insertion Probe Performance Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization Note 61: Analysis of Sugars Via a New DEP Probe Tip For Use With theDirect Probe On the HP5973 MSD Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 59: Computer Modeling of a TOF Reflectron With Gridless Reflector Using SIMION 3D Note 58: Direct Probe Analysis and Identification of Multicomponent Pharmaceutical Samples via Electron Impact MS Note 57: Aroma Profiles of Lavandula species Note 56: Mass Spec Maintenance & Cleaning Utilizing Micro-Mesh® Abrasive Sheets Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 53: SIMION 3D v6.0 Ion Optics Simulation Software Note 52: Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry Using SIMION 3D Note 51: Development and Characterization of a New Chemical Reaction Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) Note 50: The Analysis of Multiple Component Drug Samples Using a Direct Probe Interfaced to the HP 5973 MSD Note 49: Analysis of Cocaine Utilizing a New Direct Insertion Probe on a Hewlett Packard 5973 MSD Note 48: Demonstration of Sensitivity Levels For the Detection of Caffeine Using a New Direct Probe and Inlet for the HP 5973 MSD Note 47: The Application Of SIMION 6.0 To Problems In Time-of-Flight Mass Spectrometry Note 46: Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond Note 45: Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources Note 44: The Design Of a New Direct Probe Inlet For a Mass Spectrometer Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS Tech No. "A" Note 14: Elimination of "Memory" Peaks in Thermal Desorption Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers - Part I of II Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers- Part II of II Adsorbent Resins Guide Development and Field Tests of an Automated Pyrolysis Insert for Gas Chromatography. Hydrocarbon Production in Pine by Direct Thermal Extraction A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Capillary (019P) - Comparison of Septa by Direct Thermal Extraction Volatile Organic Composition in Blueberry Identification of Volatile Organic Compounds in Office Products Detection and Indentification of Volatiles in Oil Base Paintsby Headspace GC with On Column Cryo-Trapping Evaluation of Septa Using a Direct Thermal Extraction Technique INFLUENCE OF STORAGE ON BLUEBERRY VOLATILES Selection of Thermal Desorption and Cryo-Trap Parameters in the Analysis of Teas Redesign and Performance of a Diffusion Based Solvent Removal Interface for LC/MS The Design of a New Direct Probe Inlet for a Mass Spectrometer Analytes Using Mass Spectrometry (CRIMS) Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources A Student Guide for SIMION Modeling Software Application of SIMION 6.0 to Problems in Time-of-flight Mass Spectrometry Comparison of Sensitivity of Headspace GC, Purge and TrapThermal Desorption and Direct Thermal Extraction Techniques forVolatile Organics The Influence of Pump Oil Purity on Roughing Pumps Analysis of Motor Oils Using Thermal Desorption-Gas Chromatography-Mass Spectrometry IDENTIFICATION OF VOLATILE ORGANIC COMPOUNDS IN PAPER PRODUCTS Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry using SIMION 3D Seasonal Variation in Flower Volatiles Development of and Automated Microprocessor Controlled Gas chromatograph Fraction Collector / Olfactometer Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Column Design of a Microprocessor Controlled Short Path Thermal Desorption Autosampler Computer Modeling of Ion Optics in Time-of-Flight Mass Spectrometry Using SIMION 3D Thermal Desorption Instrumentation for Characterization of Odors and Flavors

- ▶
- Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption (This Page)
1Rutgers University, Center for Advanced Food Technology, 63 Dudley Road, New Brunswick, NJ 08903 : 2Scientific Instrument Services, Ringoes, NJ
Presented at PittaCon99 Meeting, Orlando, FL March, 1999
Abstract
Electron beam cured packaging samples were analyzed using an automated Short Path thermal desorption apparatus (AutoDesorb, Scientific Instrument Services, Inc., Ringoes, NJ), in conjunction with a gas chromatograph-mass spectrometer (Hewlett-Packard, Inc., Palo Alto CA). The samples were weighed and placed into custom-made glass tubes. The samples were then matrix spiked with internal standards. Next, the samples were purged of volatile products in a purge and trap sampling oven onto adsorbent resin-containing tubes (GLT tubes). The GLT tubes were then loaded into an automated Short Path thermal desorption apparatus for unattended introduction to the GC capillary column. The desorption temperature, purge gas flow rate, carrier gas flow rate, desorption temperature ramp time, and cryotrap cooling temperature are automatically controlled and can be independently defined for each sample. The volatile component profile is then identified by mass spectral analysis and quantified using internal standard methodology. Several organic components were identified including hydrocarbons from petroleum distillates, photoinitators from UV (ultraviolet) cure inks, plasticizers, and residual short chain monomers from the coating formulations. This series of analyses also serves to examine the effectiveness of the design, ruggedness, and performance of the newly developed AutoDesorb System.
INTRODUCTION
Lacquers, varnishes, and other clearcoats have traditionally been applied to various types of food packaging where they impart a glossy or semi glossy appearance. In addition to providing a topcoat, they also provide abrasion resistance and increased barrier properties. Due to their wide range of characteristics with regard to strength, barrier properties, and flexibility, their use has grown into a mainstay of the packaging industry. A major cost in manufacturing coatings, and one of the elements that has the greatest effect on quality is the cure process. The cure process is the portion of manufacture where the constituent monomers and oligomers are polymerized and crosslinked, and solvents are removed from the coating formulation.
Traditionally, the curing process has been carried out using thermal techniques. In this process, the preformed functional polymers are dissolved or dispersed in solvents. The solvent systems are either organic or water based. Organic solvent systems typically work more effectively, but have the undesirable effects of releasing high levels of VOC's into the environment and having a greater propensity for developing off odors. Oligomers, which facilitate the crosslinking process, are added along with pigments, wetting agents, flow agents, lubricants, plasticizers, polymerization aids and other compounds. The resultant formulation is then applied to a substrate and thermal energy is used to remove the solvent and to initiate the curing reaction processes. Finally, the excess heat is removed from the substrate. Thermal curing requires relatively long cure times and high energy consumption, creates residual thermal stresses, and requires expensive tooling.
An alternative to the thermal cure method is radiation curing. This technique has been in use since the 1930's. However, it was not until the late 1960's that commercial interest in the process became apparent. Radiation curing is a method of curing polymers with electron beam (EB) or ultraviolet (UV) radiation. These methods have the advantage of using solventless coating formulations, so there is no solvent removal step required. Also, curing time is on the order of seconds as opposed to hours for some thermal methods. To this date, little data exist on the VOC profile that is evolved from these coating formulations. Today, these formulations are in use on frozen dinner packaging, packaged meats, beverages, and other food and non-food applications.
The purpose of this paper is to quantify and identify volatile organic components from radiation cured packaging samples using an new automated Short Path thermal desorption GC accessory, AutoDesorb (Scientific Instrument Services, Inc.). This data can be used for quality control of the radiation cure process, determination of residual monomers, additives, and potential off-odor components. It can also be used to monitor migrants from the radiation cure process into foods, for toxicological assessment and studies regarding food contact law. Finally, the method and subsequent data set can be used to deformulate competitors' products.
Experimental Methods
Sample Preparation
Twelve samples of paperboard were clear coated with a proprietary acrylic formulation and donated by an anonymous manufacturer. From each sample a piece of 2 cm by 10 cm rectangular piece was cut. These were weighed, recorded, (approximately 400 mg each) and placed in a .25" outer diameter by 14" long glass tube and then plugged at either end with purified glass wool. The glass tube was then placed in a heated purge and trap sampling oven (Scientific Instrument Services) as shown in Figure 1. Stainless steel fittings were attached at either end of the tube to facilitate the attachment of the purge gas line at one end, and a glass lined stainless steel tube (GLT), packed with 100 mg of Tenax® TA was attached at the other end. The paperboard samples were matrix spiked with 1.0 ug of d-8 naphthalene in methanol using the solvent flush method, and were heated to 80° C for 30 minutes. During this time, a flow of 1.2 liters of helium was swept through the apparatus to sweep the released volatiles onto the GLT tubes. Additionally, eight samples of paperboard with printing and glossy clear coat were prepared as described above.
Instrumentation
The samples were thermally desorbed using the AutoDesorb System (Scientific Instrument Services, Ringoes, NJ) a novel automated Short Path thermal desorption apparatus (Figure 2). In the system, the GLT tubes that contain the sample analytes from the preparative steps above are attached to a connecting tube head and needle prior to analysis. These are then placed on the sample carousel. The connector tubes contain a ball seal that protects the sample from contamination before analysis. When analysis is ready to begin, the carousel turns to advance the first sample into place. Then a pneumatic actuator extends to lift the first sample out of the carousel. Simultaneously, the connector head docks with the pickup assembly to lock it in place and depress the ball seal. The assembly is then retracted directly in-line with the GC injection port. Next, a flow of carrier gas (80 ml) is initiated to purge the GLT tube of residual oxygen and solvent. The pneumatic piston then injects the desorption tube assembly into the GC injection port where the needle serves as the transfer line to the GC. A pair of bilaterally operating heater blocks enclose around the sample cartridge to provide rapid heat transfer to the sample. The combination of flow through the desorption tube and heat from the blocks sweep the analytes through the needle and into the GC injection port. These analytes are then trapped at the front of the GC column using a Micro Cryotrap (Scientific Instrument Services, Inc., Ringoes, NJ) which is used to cool the column to subambient temperatures. A short 20cm x .53mm diameter fused silica precolumn is attached to the Micro Cryotrap to focus the analytes in a narrow band. After the analytes have been trapped onto the front of the column and the desorption cycle is complete, the blocks open, the system uninjects the sample, and the pneumatic arm places the cartridge back onto the carousel. After the column pressure equilibrates, the temperature program begins and the cryotrap heats to the specified temperature. The AutoDesorb then advances the carousel to the next sample to be analyzed. The AutoDesorb system automatically integrates and controls all features of the system suc has: cryotrap cooling/heating, purge gas flow, thermal desorption temperature (and ramp rate if applicable), and thermal desorption time. All parameters are fully integrated with Hewlett-Packard ChemStation software so that data only needs to be entered once. The AutoDesorb logs all analysis results for error detection and reporting.
The AutoDesorb was used in conjunction with an HP 6890 gas chromatograph
and 5973 mass selective detector. The mass spectrometer was operated in
electron impact (EI) ionization mode and scanned from 35-350 Da during
the GC run for the total ion chromatogram. The total ion signal was integrated
using Hewlett-Packard ChemStation software, and each of the chromatogram
peaks was library searched utilizing the United States National Institute
for Standards and Technology (NIST) library to identify the organic compounds.
For those peaks with no library match, manual interpretation was done using
comparison to analytical reference standards, proprietary mass spectral
database, and GC retention time index. The samples were analyzed unattended,
under the following AutoDesorb and GC-MS conditions:
| AutoDesorb Conditions | |
| Initial Desorption Temperature | 250° C |
| Final Desorption Temperature | 250° C |
| Desorption Ramp Rate | 0 |
| Desorption Time | 5 minutes |
| Sample prepurge | 2 minutes |
| Initial cryotrap temperature | -65° C |
| Final cryotrap temperature | 280° C |
| GC Conditions | |
| Column | HP 35ms, 60m x 250m m x .25 m m |
| GC column initial temperature | 50° C |
| GC column final temperature | 250° C |
| GC column ramp rate | 10° C/minute |
| Injection split ratio | 100:1 |
| Mass Spectrometer Conditions | |
| MS Mode | EI |
| Mass Range | 35-350 |
| Scans/second | 2.36 |
Results and Discussion
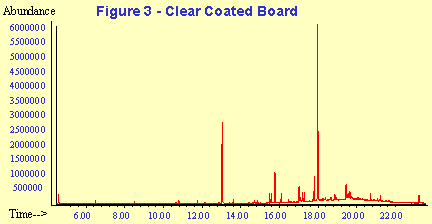
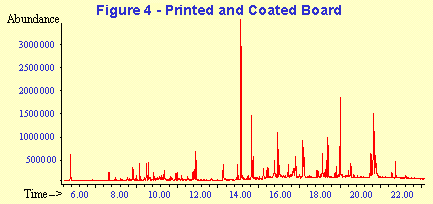
A representative GC-MS chromatogram from each of the sample types is shown in Figures 3 and 4. The peak assignments and quantitation data corresponding to these chromatograms are summarized in Tables 2 and 3. The concentration data are given in the tables in units of parts-per-billion on a weight to weight basis (PPB w/w) and in units of ug/cm2. Manufacturer's specifications are often given in mg/ream of packaging where 1 ream equals 500 square feet. Concentration data on a weight/surface area basis can then be calculated and extrapolated to mg/ream.
| Table 2 - Volatiles from Printed and Coated Paperboard Sample | |||
| Retention Time | Assignment | Concentration in PPB (w/w) | Concentration in wt/area (ng/cm2) |
| 6.69 | branched c-10 hydrocarbon | 17.2 | 0.92 |
| 7.42 | tetramethyl pentadecane | 80.9 | 4.33 |
| 10.64 | nonanal | 46.8 | 2.51 |
| 10.71 | dodecane | 56.3 | 3.01 |
| 12.14 | decanal | 46.3 | 2.48 |
| 12.18 | tridecane | 80.8 | 4.33 |
| 12.87 | cyclic polydimethyl siloxane oligomer (column artifact) | 87.3 | 4.67 |
| 13.04 | d-8 naphthalene (internal standard) | 933.7 | 50.00 |
| 13.39 | triisopropyl benzene | 80.9 | 4.33 |
| 13.57 | tetradecane | 330.2 | 17.68 |
| 13.65 | 1-tetradecene | 113.1 | 6.06 |
| 14.15 | p-tert-butyl phenol | 89.3 | 4.78 |
| 14.34 | pentadecane | 94.0 | 5.03 |
| 14.70 | Kodaflex® type plasticizer | 89.3 | 4.78 |
| 14.87 | pentadecane | 300.4 | 16.09 |
| 15.38 | methyl pentadecane | 86.2 | 4.62 |
| 15.50 | benzophenone | 154.2 | 8.26 |
| 15.76 | hexanediolmonoacrylate | 124.7 | 6.68 |
| 16.10 | hexadecane | 220.8 | 11.82 |
| 16.16 | 3-hexadecene | 166.8 | 8.93 |
| 16.19 | 1-hexadecene | 97.8 | 5.24 |
| 16.79 | 2-methyl hexadecane | 39.7 | 2.13 |
| 17.08 | diisopropyl naphthalene | 135.3 | 7.25 |
| 17.28 | heptadecane | 85.0 | 4.55 |
| 17.36 | tetradecanal | 199.3 | 10.67 |
| 17.19 | hexendioldiacrylate | 51.0 | 2.73 |
| 17.97 | hexanedioldiacrylate (HDODA) | 407.0 | 21.79 |
| 18.39 | octadecane | 35.4 | 1.90 |
| 18.50 | hexadecanal | 84.0 | 4.50 |
| 19.49 - 19.73 | tripropyleneglycoldiacrylate (TPGDA) | 747.0 | 40.00 |
| 20.53 | 2,4 diphenyl-4-methyl-1-e-pentene | 29.3 | 1.57 |
| 20.71 | trimethylolpropanetriacrylate (TMPTA) | 87.8 | 4.70 |
In the samples we see the presence of several acrylates. These are residual short chain monomers and oligomers that evolve due to incomplete curing or radiolytic decomposition products. Due to the coating formulation, we see hexanedioldiacrylate (HDODA), hexendioldiacrylate, ethoxylated hexanedioldiacrylates, and trimethylolpropanetriacrylate. In the printed sample there are several hydrocarbons such as decane, nonane and branched chain hydrocarbons. These products may arise from residual lubricants used in paperboard processing, ink solvents for printing, binders, clays, and sizing agents. In addition we see benzophenone, a photoinitiator used in UV-cure inks. They are molecules that, when light energy in a given wavelength hits it, will cleave homolytically to give a free radical. The free radical generation is to initiate and accerlate the polymerization/crosslinking chain reaction. The final class of compounds seen is phthalates, which are used as plasticizers and/or viscosity control agents.
ConclusionThe purge and trap or dynamic headspace technique followed by thermal desorption has been utilized for the identification and quantification of the volatile organic compounds in electron beam cured coating formulations. This analytical method can be used to analyze these samples unattended through the use of a new automated system. The data presented here is novel, because currently little data is publicly available on the volatiles from radiation cured packaging. The method can easily be integrated for quality assurance purposes in manufacturing facilities or buyer acceptance. Since this system has the capability to run unattended, it can be used to automatically develop methods by varying sample sizes, purge time, desorption time, cryotrap cooling temperature, and GC column temperature program. Since several samples can be analyzed, the reproducibility and precision of the data obtained can be assessed using statistical methods. The purge and trap method followed by automated Short Path thermal desorption can be applied to a variety of sample matrices, including spices, herbs, air, integrated circuits, and foods. Preliminary data with this system has been generated on hydrocarbon and antioxidant standards, deuterated surrogate internal standards, spices (saffron) and food (cookies).

