- ▶
- Heaters/Source
- ▶
- Agilent Heaters and SensorsMass Spectrometry, Scientific Supplies & ManufacturingScientific Instrument Services 5973 Source Heater Tamper Resistant Allen Wrench 5973/5975 Quad Sensor 5985 Source Heater Assembly Agilent Interface Heater Assembly 5971 Interface Heater

- ▶
- Reference Material on InstrumentationArticle - A High Temperature Direct Probe for a Mass Spectrometer Design of a Direct Exposure Probe and Controller for use ona Hewlett-Packard 5989 Mass Spectrometer SIS AP1000 AutoProbe™ SIS AP2000 AutoProbe™ - Description of System HPP7: Direct Probe Electronics Console HPP7: Direct Probe for the Agilent (HP) 5973/5975 MSD HPP7: HP Direct Probe Application Notes HPP7: Installation Directions for the Direct Probe HPP7: Side Cover for the HP 5973 MSD HPP7: Support HPP7: Probe Inlet System for the Agilent (HP) 5973 and 5975 MSD with Automatic Indexed Stops HPP7: Theory of Operation of the Direct Probe and Probe Inlet System Direct Thermal Extraction Thermal Desorption Application Notes Environmental Thermal Desorption Application Notes Food Science Thermal Desorption Application Notes Forensic Thermal Desorption Application Notes GC Cryo-Trap Application Notes Headspace Application Notes Purge & Trap Thermal Desorption Application Notes Theory of Operation of the AutoDesorb® System AutoDesorb Notes for SIS Dealers Adsorbent Resin Application Notes Installation of the Short Path Thermal Desorption System on Agilent (HP) and Other GCs Installation of the Short Path Thermal Desorption System on a Varian 3400 GC AutoDesorb® System Development Team Thermal Desorption Applications and Reference Materials Installation of the Short Path Thermal Desorption System - TD5 Part I - Design & Operation of the Short Path ThermalDesorption System Installation Instructions for the Model 951 GC Cryo-Trap on the HP 5890 Series GC Installation Instructions for the Model 961 GC Cryo-Trap on the HP 5890 Series GC Operation of the Model 951/961 GC Cryo-Trap SIS GC Cryo Traps - Theory of Operation NIST/EPA/NIH Mass Spectral Enhancements - 1998 version (NIST98) SIMION 3D Ion Optics Class Mass Spectrometer Source Cleaning Methods MS Tip: Mass Spectrometer Source Cleaning Procedures Mass Spec Source Cleaning Procedures Micro-Mesh® Abrasive Sheets Research Papers Using New Era Syringe Pump Systems EI Positive Ion Spectra for Perfluorokerosene (PFK) Cap Liner Information How do I convert between fluid oz and milliliters? Which bottle material should I choose? Which bottle mouth should I choose? The Bottle Selection Guide CGA Connections for Gas Tanks Chemical Reaction Interface Mass Spectrometry (CRIMS)

- TD
- ▶
- AccessoriesTD Supply Kit Desorption Tubes Adsorbent Resins Desorption Tube Needles Desorption Tube Seals Desorption System Fittings GC Cryo-Trap Extraction Cell TD Sample Loader Prepacked, Conditioned Desorption Tubes Desorption Tube Packing Accessories Stainless Steel Purge Heads Injection Port Liners Tenax TA Poster TD Application Notes Customer Service

- LiteratureApplication Notes Adsorbent Resins Guide Mass Spec Tips SDS Sheets FAQ MS Calibration Compound Spectra Manuals MS Links/Labs/ Organizations MS Online Tools Flyers on Products/Services Scientific Supplies Catalog About Us NextAdvance Bullet Blender® Homogenizer Protocols Micro-Mesh® Literature Instrumentation Literature Agilent GC/MS Literature SIS News / E-Mail Newsletter NIST MS Database - Update Notifications

- ▶
- Thermal Desorption Applications and Reference MaterialsDirect Thermal Extraction Headspace Environmental Food Science Applications Pharmaceuticals Forensic Note 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 57: Aroma Profiles of Lavandula species Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS

- Application NotesNote 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 96: Reducing Warping in Mass Spectrometer Filaments, with SISAlloy® Yttria/Rhenium Filaments Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 92: Yttria Coated Mass Spectrometer Filaments Note 91: AutoProbe DEP Probe Tip Temperatures Note 90: An Automated MS Direct Probe for use in an Open Access Environment Note 89: Quantitation of Organics via a Mass Spectrometer Automated Direct Probe Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 87: Design and Development of an Automated Direct Probe for a Mass Spectrometer Note 86: Simulation of a Unique Cylindrical Quadrupole Mass Analyzer Using SIMION 7.0. Note 85: Replacing an Electron Multiplier in the Agilent (HP) 5973 MSD Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 81: Rapid Bacterial Chemotaxonomy By DirectProbe/MSD Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 78: A New Solution to Eliminate MS Down-Time With No-Tool-Changing of Analytical GC Columns Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 76: Determination of the Sensitivity of a CRIMS System Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 72: 1998 Version of the NIST/EPA/NIH Mass Spectral Library, NIST98 Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 70: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part II Note 69: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part 1 Note 68: Use of a PC Plug-In UV-Vis Spectrometer To Monitor the Plasma Conditions In GC-CRIMS Note 67: Using Chemical Reaction Interface Mass Spectrometry (CRIMS) To Monitor Bacterial Transport In In Situ Bioremediation Note 66: Probe Tip Design For the Optimization of Direct Insertion Probe Performance Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization Note 61: Analysis of Sugars Via a New DEP Probe Tip For Use With theDirect Probe On the HP5973 MSD Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 59: Computer Modeling of a TOF Reflectron With Gridless Reflector Using SIMION 3D Note 58: Direct Probe Analysis and Identification of Multicomponent Pharmaceutical Samples via Electron Impact MS Note 57: Aroma Profiles of Lavandula species Note 56: Mass Spec Maintenance & Cleaning Utilizing Micro-Mesh® Abrasive Sheets Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 53: SIMION 3D v6.0 Ion Optics Simulation Software Note 52: Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry Using SIMION 3D Note 51: Development and Characterization of a New Chemical Reaction Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) Note 50: The Analysis of Multiple Component Drug Samples Using a Direct Probe Interfaced to the HP 5973 MSD Note 49: Analysis of Cocaine Utilizing a New Direct Insertion Probe on a Hewlett Packard 5973 MSD Note 48: Demonstration of Sensitivity Levels For the Detection of Caffeine Using a New Direct Probe and Inlet for the HP 5973 MSD Note 47: The Application Of SIMION 6.0 To Problems In Time-of-Flight Mass Spectrometry Note 46: Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond Note 45: Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources Note 44: The Design Of a New Direct Probe Inlet For a Mass Spectrometer Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS Tech No. "A" Note 14: Elimination of "Memory" Peaks in Thermal Desorption Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers - Part I of II Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers- Part II of II Adsorbent Resins Guide Development and Field Tests of an Automated Pyrolysis Insert for Gas Chromatography. Hydrocarbon Production in Pine by Direct Thermal Extraction A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Capillary (019P) - Comparison of Septa by Direct Thermal Extraction Volatile Organic Composition in Blueberry Identification of Volatile Organic Compounds in Office Products Detection and Indentification of Volatiles in Oil Base Paintsby Headspace GC with On Column Cryo-Trapping Evaluation of Septa Using a Direct Thermal Extraction Technique INFLUENCE OF STORAGE ON BLUEBERRY VOLATILES Selection of Thermal Desorption and Cryo-Trap Parameters in the Analysis of Teas Redesign and Performance of a Diffusion Based Solvent Removal Interface for LC/MS The Design of a New Direct Probe Inlet for a Mass Spectrometer Analytes Using Mass Spectrometry (CRIMS) Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources A Student Guide for SIMION Modeling Software Application of SIMION 6.0 to Problems in Time-of-flight Mass Spectrometry Comparison of Sensitivity of Headspace GC, Purge and TrapThermal Desorption and Direct Thermal Extraction Techniques forVolatile Organics The Influence of Pump Oil Purity on Roughing Pumps Analysis of Motor Oils Using Thermal Desorption-Gas Chromatography-Mass Spectrometry IDENTIFICATION OF VOLATILE ORGANIC COMPOUNDS IN PAPER PRODUCTS Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry using SIMION 3D Seasonal Variation in Flower Volatiles Development of and Automated Microprocessor Controlled Gas chromatograph Fraction Collector / Olfactometer Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Column Design of a Microprocessor Controlled Short Path Thermal Desorption Autosampler Computer Modeling of Ion Optics in Time-of-Flight Mass Spectrometry Using SIMION 3D Thermal Desorption Instrumentation for Characterization of Odors and Flavors

- ▶
- Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption (This Page)
* Scientific Instrument Services, Ringoes, NJ
Department of Food Science, Cook College, Rutgers
University, New Brunswick, NJ 08903
† Center for Advanced Food Technology, Cook College,
Rutgers University, New Brunswick, NJ 08903
Presented at EAS, Somerset, NJ., November 1998
Abstract
Most headspace analysis of plant material is performed on culled or harvested plant parts.1,2 While adequate for most flavor and fragrance work (as these parts are the source of commercial additives and extracts), the act of harvesting the material can introduce artifacts that interfere with the study of normal biological activity. Ideally, the examination volatiles should be undertaken on living undisturbed plants because stresses, as physical damage or temperature fluctuations, can cause dramatic changes in the quality and quantity of volatile emissions.3
This work describes the design and operation of an apparatus for the collection of volatile organics from living plants. Attention was focused on minimizing physical damage to the plant being sampled, and eliminating artifacts arising from materials included in the apparatus itself. The equipment consists mainly of a specially made split glass flask that can surround the plant or plant part to be sampled and provide a known volume for quantitative calculations. Associated equipment includes a vacuum pump, flow control devices, a reservoir of clean air to replace the sampled headspace, adsorbent traps for retaining the sample, and a means of sealing the apparatus. Data were gathered from several tomato plants at different stages of development, and rates of emission for volatile organics are shown. Analysis was by Thermal Desorption/Gas Chromatography (TD/GC) with qualitative confirmation using retention indices and Mass Spectrometry. The overwhelming majority of the compounds detected from live tomato plants were terpenoids, and rates of total organic emissions varied with plant age.
Background
Static and dynamic headspace sampling have been applied routinely to the analysis of volatile compounds from plant tissue. These methods represent the most direct way of qualitatively and quantitatively measuring the constituents of particular plant aromas as well as non odor-active components of volatile plant emissions. Flavor and fragrance researchers may be the most frequent users of these techniques, however they are also used more and more often by those investigating biologically active principals as well as individuals interested in the environmental impact of volatiles from plants.1,2 An example of the latter type would include the study of the contribution of plant volatiles to changes in both in- and outdoor air quality, while the former includes measurement of plant hormones and signaling agents, as ethylene and methyl salicylate.4,5
The focus of this work is to develop a sampling system that can accurately reflect the volatile material emitted by living plants, without introducing sampling artifacts by damaging them. Plants can react to stress by altering the type and amount of volatile material they emit, as in the case of Solanacea releasing volatile terpenes when attacked by insects, or Tobacco plants emitting Methyl Salicylate after being infected with Tobacco Mosaic Virus.6,5 Most plant tissues begin to break down enzymatically soon after harvest. Some enzymes are particularly effective at liberating volatile material from lipids, for example. Retaining the integrity of the live plant is therefore crucial to characterizing its normal volatile profile. Another objective of the work is refining the sampling methodology to eliminate background contamination and obtain cleaner samples. Many of the systems in use for collecting plant volatiles are inadequate not only because they require the vegetation to be harvested prior to sampling, but they are also constructed of materials that are incompatible with artifact-free analysis. Plastics, in particular, are often used in purging vessels and for tubing to direct airflow, etc. Stabilizers and other volatile additives are common artifacts in samples from such systems. By sampling living plants directly with a system made of inert materials, better data can be obtained for environmental as well as biological research. Direct measurement of the rate of volatile emissions, for example, is of interest to the space program, where plants will eventually be included in self-sustaining life support systems.7
Experimental
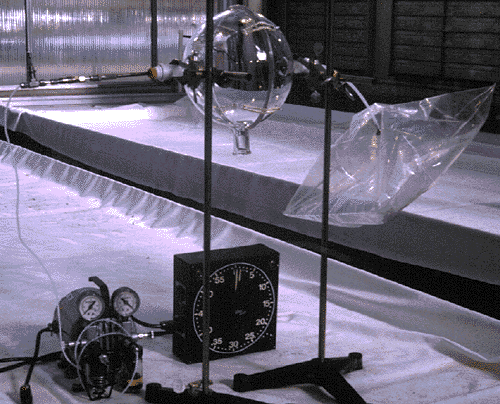
Figure 1
Custom manufactured glass enclosures (Kimble, Inc. Vineland,NJ) are used to surround the plant part to be sampled. The enclosure is made of two identical hemispherical parts with a ground glass sealing surface where they mate. An opening along the edge of each hemisphere allows the stem of the sample to protrude and remain attached to the rest of the plant throughout the sampling procedure. A size 24/40 tapered ground glass sampling port is located on each half of the enclosure and when the unit is assembled, these ports are directly opposite one another. The nominal volume of the enclosures used in most of the experiments was 5000 ml. This value was corrected for the volume taken up by the sample (see Calculations, below). The enclosure is supported by ringstand clamps, and is sealed around the plant stem with PTFE tape or Parafilm. These materials have been evaluated and found to be free of volatile components that might contaminate the sample at the temperatures normally encountered. Figures 1&2 illustrate the apparatus empty, and while sampling respectively.
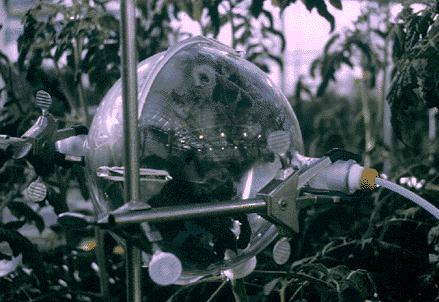
Figure 2
Air samples are drawn from the enclosure through one of the sampling ports. The air is drawn by a vacuum pump (Fisher Scientific) and is regulated by a fine metering valve (Nupro Inc.). The sample rate is 100 ml/minute and is verified before and after sampling with a digital flowmeter (Alltech Inc.). Sampled air is replaced from a 10 liter Tedlar reservoir (SKC Eighty-four, PA) filled with compressed air (JWS Piscataway, NJ) conditioned with a hydrocarbon trap (Supelco Bellefonte, PA). The samples are drawn through a glass-lined stainless steel desorption tube filled with 100 mg Tenax® TA (SIS Inc. Ringoes, NJ). The tubes are tightly capped immediately after sampling, and the plant part is severed at a point even with the edge of the enclosure and dried at 70 ºC for 48 hours before being weighed.
Two sets of plants were evaluated over a period of approximately 10 weeks. Duplicate series of five samples were taken at intervals of 7 to 10 days beginning at 25 days after planting (DAP) and continuing through approximately 75-80 DAP in each experiment.
Analysis
Sampling tubes were spiked with an internal standard solution of approximately 1.0 µg/µl each d-8 toluene and d-8 naphthalene in methanol. One tube from each sample set was spiked at a higher level of approximately 10.0µg/µl to allow estimation of higher sample concentrations. The tubes were purged for approximately 15 minutes with 30 ml/min dry nitrogen to remove solvent from the standard spike and water.
Samples were analyzed with a SIS model TD-1 Short Path Thermal Desorption system on a Varian 3400 Gas Chromatograph (GC). Detection was by Flame Ionization (FID), and data were collected using a Varian Star chromatography data system version 4.0. GC parameters are summarized below:
Column: 60 m DB-1 (J&W Folsom, CA) 0.53 mm, 0.5µm film thickness
Injector : Split 25:1 or 300:1, Temperature: 250 ºC
Detector: FID, Temperature: 250 ºC
Desorption Conditions: 250 ºC for 5 minutes
Oven Program:
Initial: -20 ºC; 0 minutes
Ramp: 10 ºC /minute
Final: 280 ºC; 5 minutes
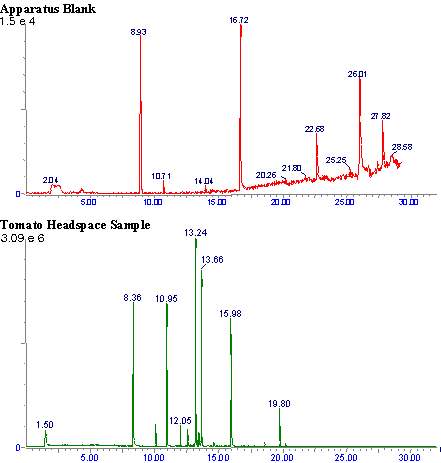
Figure 3
Typical chromatograms of an apparatus blank, and a tomato headspace sample are shown in Figure 3.
Gas chromatography-Mass spectrometry (GC-MS) was used for qualitative confirmation of one sample from each set. GC parameters were identical to those used for FID detection, except the column was 0.25 mm, 0.25 µm film thickness; and the sample was split 100:1 at the injector. A Finnigan MAT model 8230 mass spectrometer with a Finnigan SSX data system was used for MS detection. Electron Impact (EI) ionization (70 eV) was used, and the instrument was set to scan from 35 to 350 amu at 0.1 seconds per decade, with an interscan time of 0.8 seconds.
Standards containing hexanal, p-cymene, limonene, terpineol, and isocaryophyllene in addition to internal standards were spiked onto packed desorption tubes, purged in the same manner that the samples were, and then analyzed to create five standard curves. With the exception of p-cymene, which was saturated at the higher spike levels, all the curves had similar slopes, and all had response factors close to unity. The chromatograms were therefore calculated with a single response factor of 1.0 relative to the d-8 toluene internal standard, and the results should be considered semi-quantitative.
Table 1. Results of a Typical Tomato Headspace
Analysis
| Ret. Time | Area | RRt | Ret. Index | ng | Compound |
| 6.35 | 1990 | -1.54 | unknown | 3 | unknown |
| 7.89 | 655827 | 0 | 748 | 991 | Internal Standard |
| 8.57 | 8865 | 0.67 | 771 | 13.4 | toluene |
| 9.65 | 7526 | 1.76 | 812 | 11.38 | silicone |
| 9.9 | 11636 | 2.01 | 822 | 17.58 | unkown |
| 10.42 | 1320 | 2.53 | 843 | 1.99 | unk. terpene |
| 10.78 | 2785 | 2.88 | 858 | 4.21 | aliphatic HC |
| 11.55 | 1722 | 3.66 | 891 | 2.61 | unk. terpene |
| 11.69 | 140256 | 3.8 | 897 | 211.94 | alpha pinene |
| 12.27 | 163657 | 4.38 | 923 | 247.29 | cymene isomer |
| 12.35 | 1024 | 4.46 | 927 | 1.55 | cymene isomer |
| 12.44 | 3255 | 4.54 | 930 | 4.92 | sabinene |
| 12.64 | 10222 | 4.75 | 940 | 15.45 | 2-carene |
| 12.89 | 1113639 | 4.99 | 951 | 1682.79 | alpha phellandrene |
| 13.02 | 1359 | 5.13 | 957 | 2.05 | carene isomer |
| 13.06 | 1303 | 5.16 | 959 | 1.97 | carene isomer |
| 13.13 | 68050 | 5.24 | 963 | 102.83 | alpha terpinene |
| 13.34 | *4761605 | 5.45 | 972 | *7195.12 | terpinene isomers + limonene |
| 13.5 | 1224 | 5.61 | 980 | 1.85 | limonene + cis-ocimene |
| 13.62 | 1841 | 5.73 | 986 | 2.78 | ocimene |
| 13.84 | 5060 | 5.95 | 996 | 7.65 | gamma terpinene |
| 14.36 | 11897 | 6.46 | 1021 | 17.98 | dimethyl styrene isomer |
| 14.85 | 2414 | 6.96 | 1046 | 3.65 | oxygenated terpene |
| 15.33 | 1047 | 7.43 | 1070 | 1.59 | 2,6 dimethyl styrene |
| 15.7 | 633456 | 7.8 | 1090 | 957.2 | Naphthalene I.S. |
| 16.33 | 1319 | 8.43 | 1122 | 1.99 | 1-decene |
| 18.39 | 2046 | 10.5 | 1235 | 3.09 | delta elemene |
| 19.63 | 6167 | 11.73 | 1307 | 9.32 | isocaryophyllene |
| 19.97 | 1004 | 12.07 | 1327 | 1.52 | unk sesquiterpenes |
| 21.67 | 1373 | 13.77 | 1420 | 2.07 | unknown |
| 30.78 | 1067 | 22.88 | unknown | 1.62 | unknown |
| 32.48 | 4284 | 24.59 | unknown | 6.47 | unknown |
| Total ng in tube: 9570.3 | |||||
| Total ng in chamber: 232796.5 | |||||
| Total ng per g Foliage: 17244.2 | |||||
| Emission Rate per gram: 20.7 | |||||
| (Rate in m g/hr/g dry weight) | |||||
| mid-sample interval = 5 minutes | |||||
| * Corrected | |||||
Calculations
The d-8 toluene internal standard was used as a retention time reference, because it is an easily recognizable peak in the chromatograms, and its retention time was also very stable throughout the experiments. Relative retention times were calculated for all peaks with over 1000 area counts by dividing the retention time of each peak by that of the toluene internal standard. Peaks with areas under 1000 counts were below the quantitative limit of the method. A standard containing thirteen straight-chain hydrocarbons was run using the same GC oven program as the samples, and retention indices were generated using the relative retention times. The retention indices were used to help correlate the peaks in the GC chromatograms with those that could be identified in the GC-MS runs. The d-8 naphthalene internal standard was used to correct any minor spread in the retention indices by observing its relationship to the toluene internal standard.
Because of the large quantities of some individual compounds found in the tomato headspace, it was necessary to spike two different levels of internal standards and to run one sample from each set at a higher split to avoid overloading the largest peaks. In this way, the dynamic range of the method was extended to include compounds that are present in the highest quantity, without sacrificing sensitivity to those present at trace levels. In order to perform accurate calculations for total organic emissions, the areas of overloaded peaks must be estimated in the low-range chromatograms by using a ratio of some other peak from the sample to the peak in question. The peak to be used in the ratio must be present and on scale in both high- and low-range chromatograms. In these experiments, the one peak that was consistently overloaded was an isomer of terpinene mixed with lower levels of limonene. The peak used as a reference was identified as a -phellandrene. Care was taken to use the ratio from the same plant and sampling interval when possible.
Once all the peaks in the chromatogram had been quantified, summing the amounts of individual compounds, exclusive of internal standards and known artifacts, gave the total amount of organic material by weight that was trapped on the adsorbent tube. This value was applied to the sample volume (generally 200 ml) to yield the concentration for that sampling interval. This was considered equivalent to an instantaneous concentration at the mid-point of the sample. The time between the onset of sampling (t0) and the midpoint of a sample was termed the 'mid-sample interval' for that sample. Thus, since the samples were uniformly 2 minutes long, the mid-sample interval (in minutes) for any consecutively numbered sample 'n' was equal to 2n-1. The concentration at the mid-sample interval was then applied to the volume of the sampling enclosure after it had been corrected for the space taken up by the sample itself. This figure, when divided by the mid-sample interval, yields an emission rate in mg/min. The emission rate per gram of sample is easily calculated.
Results
The samples from experimental Set I displayed total organic emission in the range of approximately 12 to 120 m g/hour/g, with an average value of 49.15 m g/hour/g. An apparent periodic trend, with maxima at 40 and approximately 70 days (coinciding with plant flowering and fruit ripening respectively) was not confirmed by the second set of samples. Set II showed a more or less steady decline in emission rate throughout the life of the plants, with somewhat lower emission rates between 5 and 70 m g/hour/g, and averaging 29.55 m g/hour/g. A foundation for fluctuations in the emission rate remains elusive, although environmental factors such as temperature and humidity can be investigated by plotting both graphs against the date (the experiments were not run wholly concurrently). The emission rates are plotted against plant age in Figure 4.
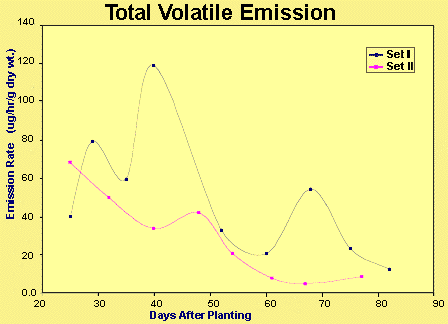
Figure 4
As is apparent from the blank chromatogram, (Figure 3.) the apparatus is free from interfering contaminants. The two large peaks are the internal standards, and the three smaller peaks have been identified as siloxane compounds from the GC column. The apparatus, however, may still contribute to plant stress by inhibiting transpiration. It was noted that the relative humidity in the chamber routinely rose to near saturation during sampling. The entire sampling interval, however, lasted only 10 minutes.
Conclusions
A simple, rugged device has been developed that can aid in the analysis of volatile components emitted by live plants. In duplicate experiments, the apparatus allowed the collection of data that yielded emission rates for total volatile organic material from live tomatoes. The chromatographic data was largely free from interference related to the sampling apparatus. Much information remains in the data, and future work will examine relationships between plant age and qualitative sample composition as well as the potential for volatile compounds to be used as monitors of plant stress.
Acknowledgments
This work was funded by the New Jersey - NASA Specialized Center of
Research and Training (NJ-NSCORT).
References
1. Bicchi, C. and Joulain, D. 1990. Headspace Gas Chromatographic Analysis of Medicinal and Aromatic Plants and Flowers. Flavor and Fragrance Journal 5, 131-145.
2. Buttery, R.G., Ling, L.C. and Light, D.M. 1987. Tomato leaf Volatile Aroma Components. J. Agric. Food Chem. 35, 1039-1040.
3. Heath, R.R., and Manukian, A. 1994 An Automated System for use in Collecting Volatile Chemicals Released from Plants. J. Chemical Ecology 20:3,593-608.
4. Batten, J.H., Stutte, G.W. and Wheeler, R.M. 1996. Volatile Organic compounds Detected in the Atmosphere of NASA's Biomass Production Chamber. Adv. Space Res. 18:4/5, 189-192.
5. Shulaev, V., Silverman, P.and Raskin , I. 1997. Airborne Signalling by Methyl Salicylate In Plant Pathogen Resistance. Nature. 320, 718-721.
6. Alborn, H.T., Turlings, T.C.J., Jones, T.H., Stenhagen, G., Loughrin, J.H. and Tumlinson, J.H. 1997. An Elicitor of Plant Volatiles from Beet Armyworm Oral Secretion. Science. 276, 945-949.
7. Lange, K.E., Lin, C.H. and Barnes, C. 1996 Advanced Life Support Program Requirements Definition and Technology Development Needs (Preliminary). Document no. CTSD-ADV-XXX. Crew and Thermal Systems Division, NASA Johnson Space Center, Houston, Texas. A1-A4.

