- ▶
- Heaters/Source
- ▶
- Agilent Heaters and SensorsMass Spectrometry, Scientific Supplies & ManufacturingScientific Instrument Services 5973 Source Heater Tamper Resistant Allen Wrench 5973/5975 Quad Sensor 5985 Source Heater Assembly Agilent Interface Heater Assembly 5971 Interface Heater

- ▶
- SISWEB Application NotesNote 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 96: Reducing Warping in Mass Spectrometer Filaments, with SISAlloy® Yttria/Rhenium Filaments Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 92: Yttria Coated Mass Spectrometer Filaments Note 91: AutoProbe DEP Probe Tip Temperatures Note 90: An Automated MS Direct Probe for use in an Open Access Environment Note 89: Quantitation of Organics via a Mass Spectrometer Automated Direct Probe Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 87: Design and Development of an Automated Direct Probe for a Mass Spectrometer Note 86: Simulation of a Unique Cylindrical Quadrupole Mass Analyzer Using SIMION 7.0. Note 85: Replacing an Electron Multiplier in the Agilent (HP) 5973 MSD Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 81: Rapid Bacterial Chemotaxonomy By DirectProbe/MSD Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 78: A New Solution to Eliminate MS Down-Time With No-Tool-Changing of Analytical GC Columns Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 76: Determination of the Sensitivity of a CRIMS System Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 72: 1998 Version of the NIST/EPA/NIH Mass Spectral Library, NIST98 Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 70: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part II Note 69: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part 1 Note 68: Use of a PC Plug-In UV-Vis Spectrometer To Monitor the Plasma Conditions In GC-CRIMS Note 67: Using Chemical Reaction Interface Mass Spectrometry (CRIMS) To Monitor Bacterial Transport In In Situ Bioremediation Note 66: Probe Tip Design For the Optimization of Direct Insertion Probe Performance Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization Note 61: Analysis of Sugars Via a New DEP Probe Tip For Use With theDirect Probe On the HP5973 MSD Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 59: Computer Modeling of a TOF Reflectron With Gridless Reflector Using SIMION 3D Note 58: Direct Probe Analysis and Identification of Multicomponent Pharmaceutical Samples via Electron Impact MS Note 57: Aroma Profiles of Lavandula species Note 56: Mass Spec Maintenance & Cleaning Utilizing Micro-Mesh® Abrasive Sheets Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 53: SIMION 3D v6.0 Ion Optics Simulation Software Note 52: Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry Using SIMION 3D Note 51: Development and Characterization of a New Chemical Reaction Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) Note 50: The Analysis of Multiple Component Drug Samples Using a Direct Probe Interfaced to the HP 5973 MSD Note 49: Analysis of Cocaine Utilizing a New Direct Insertion Probe on a Hewlett Packard 5973 MSD Note 48: Demonstration of Sensitivity Levels For the Detection of Caffeine Using a New Direct Probe and Inlet for the HP 5973 MSD Note 47: The Application Of SIMION 6.0 To Problems In Time-of-Flight Mass Spectrometry Note 46: Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond Note 45: Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources Note 44: The Design Of a New Direct Probe Inlet For a Mass Spectrometer Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS Tech No. "A" Note 14: Elimination of "Memory" Peaks in Thermal Desorption Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers - Part I of II Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers- Part II of II Adsorbent Resins Guide Development and Field Tests of an Automated Pyrolysis Insert for Gas Chromatography. Hydrocarbon Production in Pine by Direct Thermal Extraction A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Capillary (019P) - Comparison of Septa by Direct Thermal Extraction Volatile Organic Composition in Blueberry Identification of Volatile Organic Compounds in Office Products Detection and Indentification of Volatiles in Oil Base Paintsby Headspace GC with On Column Cryo-Trapping Evaluation of Septa Using a Direct Thermal Extraction Technique INFLUENCE OF STORAGE ON BLUEBERRY VOLATILES Selection of Thermal Desorption and Cryo-Trap Parameters in the Analysis of Teas Redesign and Performance of a Diffusion Based Solvent Removal Interface for LC/MS The Design of a New Direct Probe Inlet for a Mass Spectrometer Analytes Using Mass Spectrometry (CRIMS) Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources A Student Guide for SIMION Modeling Software Application of SIMION 6.0 to Problems in Time-of-flight Mass Spectrometry Comparison of Sensitivity of Headspace GC, Purge and TrapThermal Desorption and Direct Thermal Extraction Techniques forVolatile Organics The Influence of Pump Oil Purity on Roughing Pumps Analysis of Motor Oils Using Thermal Desorption-Gas Chromatography-Mass Spectrometry IDENTIFICATION OF VOLATILE ORGANIC COMPOUNDS IN PAPER PRODUCTS Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry using SIMION 3D Seasonal Variation in Flower Volatiles Development of and Automated Microprocessor Controlled Gas chromatograph Fraction Collector / Olfactometer Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Column Design of a Microprocessor Controlled Short Path Thermal Desorption Autosampler Computer Modeling of Ion Optics in Time-of-Flight Mass Spectrometry Using SIMION 3D Thermal Desorption Instrumentation for Characterization of Odors and Flavors

- TD
- ▶
- AccessoriesTD Supply Kit Desorption Tubes Adsorbent Resins Desorption Tube Needles Desorption Tube Seals Desorption System Fittings GC Cryo-Trap Extraction Cell TD Sample Loader Prepacked, Conditioned Desorption Tubes Desorption Tube Packing Accessories Stainless Steel Purge Heads Injection Port Liners Tenax TA Poster TD Application Notes Customer Service

- ▶
- Adsorbent Resins Guide (This Page)
Adsorbent Resins are utilized by Purge and Trap (P&T) and other thermal desorption applications. A wide variety of adsorbent resins are available from many suppliers and manufacturers. In order to properly select an adsorbent resin for a particular application the user must have knowledge of the types of analytes that he wishes to analyze as well as an understanding of the physical properties of the adsorbent resins available. This is the accumulation of about 2 years work by Scientific Instrument Services to collect and compile this data.
The data herein is designed for use by the scientific community to aid in the use of adsorbent resins. As such you are free to take and use this data for you experimentation. However the tables, charts and data enclosed herein cannot be reproduced in publications or other information sources including the Internet without the written consent of SIS. SIS will grant the right to republish this data as long as it is in the best interests of SIS and that SIS receives prominent recognition as the source of this information.
| Tenax® TA | is a porous polymer based on 2,6-diphenylene oxide. It has been specifically designed for the trapping of volatiles and semi-volatiles from liquid or solid matrices. Both the EPA and NIOSH specify the use of Tenax in their standard methods. Tenax TA is a low bleeding material with a low level of impurities. Due to its low affinity for water, Tenax TA is especially useful for the purging of volatile organics from high moisture content samples including the analysis of volatiles organics in water. |
|---|---|
| Tenax® GR | is a composite material of Tenax TA and 30% graphite. The resulting material gives a higher breakthrough volume for most volatile organics, yet still has a low affinity for water. In addition Tenax GR maintains its high temperature stability to 350 degrees C. These properties make Tenax GR an ideal adsorbent for the trapping of volatiles from air, water and solid matrices. Due to its low affinity for water, Tenax GR is especially useful for the purging of volatile organics from high moisture content samples including the analysis of volatiles organics in water. |
| Carbotrap | graphitized carbon blacks are ideal adsorbents for trapping a wide range of airborne organic compounds from butane/pentane hydrocarbons through the PCB's. It is usable to 400 degrees C and due to its coarse mesh size will provide minimal backpressure in the desorption tubes. Manufactured by Supelco. |
| Carbotrap C | graphitized carbon blacks is an ideal resin for the trapping of a wide range of organic volatiles. It also has a coarse mesh size and can be used to 400 degrees C. Manufactured by Supelco. |
| Carboxen 569 | is a carbon molecular sieve which is ideal for the trapping of the smaller organic analytes. It has a low affinity for water and can be used to trap many C2-C5 volatile organics. It is often used in conjunction with Tenax TA to produce mixed bed resins. Manufactured by Supelco. |
| Carbosieve SIII | is a carbon molecular sieve that has the greatest ability for the trapping of the low molecular weight organics due to its large surface area. |
| Glass Beads | are most useful for the trapping of large molecular weight compounds. It will not retain any of the light volatiles, aromatics or other medium boilers. Therefore it is most frequently used in mixed bed applications with other adsorbent resins. Available from Supelco. |
Adsorbent Resins Available
 Tenax® TA Adsorbent Resin Tenax® TA is a porous polymer resin based on 2.6-diphenylene oxide.
Tenax® TA Adsorbent Resin Tenax® TA is a porous polymer resin based on 2.6-diphenylene oxide. Tenax®-GR Adsorbent Resin for Trapping Volatiles The new Tenax® GR is a composite material of Tenax® TA and 30% graphite.
Tenax®-GR Adsorbent Resin for Trapping Volatiles The new Tenax® GR is a composite material of Tenax® TA and 30% graphite. Carbotrap Adsorbent Resin Physical Properties See also Carbotrap Breakthrough Volume Data Carbotrap and Carbotrap C are graphitized carbon blacks that are ideal adsorbent resins for the trapping of a wide range of organic analytes from C4/C5 through the medium boilers. They have a coarse mesh size (20/40) which prevent high backpressures in the desorption tubes Properties Chemical Structure or Name: graphitized carbon black Temperature Limit: 400 degrees C Affinity for Water: relatively low Specific Surface Area: 100 square meter per gram ...
Carbotrap Adsorbent Resin Physical Properties See also Carbotrap Breakthrough Volume Data Carbotrap and Carbotrap C are graphitized carbon blacks that are ideal adsorbent resins for the trapping of a wide range of organic analytes from C4/C5 through the medium boilers. They have a coarse mesh size (20/40) which prevent high backpressures in the desorption tubes Properties Chemical Structure or Name: graphitized carbon black Temperature Limit: 400 degrees C Affinity for Water: relatively low Specific Surface Area: 100 square meter per gram ... Carbotrap C Adsorbent Resin Physical Properties See also Carbotrap C Breakthrough Volume Data Carbotrap and Carbotrap C are graphitized carbon blacks that are ideal adsorbent resins for the trapping of a wide range of organic analytes from C4/C5 through the medium boilers. They have a coarse mesh size (20/40) which prevent high backpressures in the desorption tubes Properties Chemical Structure or Name: graphitized carbon black Temperature Limit: 400 degrees C Affinity for Water: relatively low Specific Surface Area: 100 square meter per gra...
Carbotrap C Adsorbent Resin Physical Properties See also Carbotrap C Breakthrough Volume Data Carbotrap and Carbotrap C are graphitized carbon blacks that are ideal adsorbent resins for the trapping of a wide range of organic analytes from C4/C5 through the medium boilers. They have a coarse mesh size (20/40) which prevent high backpressures in the desorption tubes Properties Chemical Structure or Name: graphitized carbon black Temperature Limit: 400 degrees C Affinity for Water: relatively low Specific Surface Area: 100 square meter per gra... Carboxen 569 Adsorbent Resin Physical Properties See also Carboxen 569 Breakthrough Volume Data Carboxen 569 is a carbon molecular sieve adsorbent resin designed for small chlorinated and other molecules. It has a large mesh size which minimizes the backpressure in the desorption tubes and also has a low affinity for water. Properties Chemical Structure or Name: carbon molecular sieve Temperature Limit: 400 degrees C Affinity for Water: relatively low Mesh size: 20/45 mesh Available from Supelco, Inc. Supelco Park, Bellefonte, PA 16823 Phone:...
Carboxen 569 Adsorbent Resin Physical Properties See also Carboxen 569 Breakthrough Volume Data Carboxen 569 is a carbon molecular sieve adsorbent resin designed for small chlorinated and other molecules. It has a large mesh size which minimizes the backpressure in the desorption tubes and also has a low affinity for water. Properties Chemical Structure or Name: carbon molecular sieve Temperature Limit: 400 degrees C Affinity for Water: relatively low Mesh size: 20/45 mesh Available from Supelco, Inc. Supelco Park, Bellefonte, PA 16823 Phone:... Carbsieve SIII Adsorbent Resin Physical Properties See also Carbosieve SIII Breakthrough Volume Data Carbosieve SIII is a large surface area carbon molecular sieve which is ideal for trapping the smallest organics. While it has a higher affinity for water than most other resins, its small pore size provides the best trapping ability for the volatile organics. Properties Chemical Structure or Name: carbon molecular sieve Temperature Limit: 400 degrees C Surface Area: 820 square meters per gram Average Pore Size; 15-40 angstrom Affinity for Water...
Carbsieve SIII Adsorbent Resin Physical Properties See also Carbosieve SIII Breakthrough Volume Data Carbosieve SIII is a large surface area carbon molecular sieve which is ideal for trapping the smallest organics. While it has a higher affinity for water than most other resins, its small pore size provides the best trapping ability for the volatile organics. Properties Chemical Structure or Name: carbon molecular sieve Temperature Limit: 400 degrees C Surface Area: 820 square meters per gram Average Pore Size; 15-40 angstrom Affinity for Water... Glass Beads Adsorbent Resin Physical Properties See also Glass Beads Breakthrough Volume Data Glass Beads provide minimal rapping ability for most analytes. They are mainly useful for the trapping of very large hydrocarbons that condense on the glass beads surface during the collection process and are readily released in the desorption process. Properties Chemical Structure or Name: glass beads Temperature Limit: 350 degrees C Average Pore Size; 250 um Affinity for Water: low Available from Supelco, Inc. Supelco Park, Bellefonte, PA 16823 Ph...
Glass Beads Adsorbent Resin Physical Properties See also Glass Beads Breakthrough Volume Data Glass Beads provide minimal rapping ability for most analytes. They are mainly useful for the trapping of very large hydrocarbons that condense on the glass beads surface during the collection process and are readily released in the desorption process. Properties Chemical Structure or Name: glass beads Temperature Limit: 350 degrees C Average Pore Size; 250 um Affinity for Water: low Available from Supelco, Inc. Supelco Park, Bellefonte, PA 16823 Ph...
Determination and Use of Breakthrough Volume Data
 Determination and Use of Breakthrough Volume Data A number of technical papers are presented here which describe how the breakthrough volume data and backpressure data on adsorbent resins was determined and how it can be used by the analyst to aid in the selection of the appropriate resin for a particular application.
Determination and Use of Breakthrough Volume Data A number of technical papers are presented here which describe how the breakthrough volume data and backpressure data on adsorbent resins was determined and how it can be used by the analyst to aid in the selection of the appropriate resin for a particular application. Tenax® TA Breakthrough Volume Data Tenax TA is a porous polymer resin based on 2,6-diphenylene-oxide.
Tenax® TA Breakthrough Volume Data Tenax TA is a porous polymer resin based on 2,6-diphenylene-oxide.
Breakthrough Volume Data By Resin
 Tenax® TA Breakthrough Volume Data Tenax TA is a porous polymer resin based on 2,6-diphenylene-oxide.
Tenax® TA Breakthrough Volume Data Tenax TA is a porous polymer resin based on 2,6-diphenylene-oxide.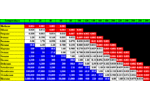 Tenax® GR Breakthrough Volume Data Tenax® GR is a porous polymer resin based on 2,6-diphenylene-oxide plus 30% Graphite.
Tenax® GR Breakthrough Volume Data Tenax® GR is a porous polymer resin based on 2,6-diphenylene-oxide plus 30% Graphite.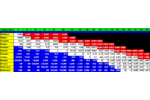 Carbotrap Breakthrough Volume Data Carbotrap with ... Hydrocarbons Alcohols & Glycols Halogens Carbotrap is a Carbon based Adsorbent Resin. It is available from Supelco. Inc. Carbotrap Breakthrough Volume Data Carbotrap with Hydrocarbons Carbotrap with Alcohols & Glycols Carbotrap with Halogens
Carbotrap Breakthrough Volume Data Carbotrap with ... Hydrocarbons Alcohols & Glycols Halogens Carbotrap is a Carbon based Adsorbent Resin. It is available from Supelco. Inc. Carbotrap Breakthrough Volume Data Carbotrap with Hydrocarbons Carbotrap with Alcohols & Glycols Carbotrap with Halogens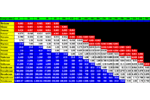 Carbotrap C Breakthrough Volume Data Carbotrap C with ... Hydrocarbons Alcohols & Glycols Halogens Carbotrap C is a Carbon based Adsorbent Resin. It is available from Supelco. Inc. Carbotrap C Breakthrough Volume Data Carbotrap C with Hydrocarbons Carbotrap C with Alcohols & Glycols Carbotrap C with Halogens
Carbotrap C Breakthrough Volume Data Carbotrap C with ... Hydrocarbons Alcohols & Glycols Halogens Carbotrap C is a Carbon based Adsorbent Resin. It is available from Supelco. Inc. Carbotrap C Breakthrough Volume Data Carbotrap C with Hydrocarbons Carbotrap C with Alcohols & Glycols Carbotrap C with Halogens Carboxen 569 Breakthrough Volume Data Carboxen 569 with ... Hydrocarbons Alcohols Aldehydes and Ketones Halogens Amines Aromatics and Terpenes Carboxen 569 is a carbon based adsorbent Resin. It is available from Supelco, Inc. Carboxen 569 Breakthrough Volume Data Carboxen 569 with Hydrocarbons Carboxen 569 with Alcohols Carboxen 569 with Aldehydes and Ketones Carboxen 569 with Halogens Carboxen 569 with Amines Carboxen 569 with Aromatics and Terpenes
Carboxen 569 Breakthrough Volume Data Carboxen 569 with ... Hydrocarbons Alcohols Aldehydes and Ketones Halogens Amines Aromatics and Terpenes Carboxen 569 is a carbon based adsorbent Resin. It is available from Supelco, Inc. Carboxen 569 Breakthrough Volume Data Carboxen 569 with Hydrocarbons Carboxen 569 with Alcohols Carboxen 569 with Aldehydes and Ketones Carboxen 569 with Halogens Carboxen 569 with Amines Carboxen 569 with Aromatics and Terpenes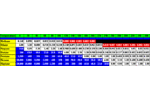 Carbosieve SIII Breakthrough Volume Data Carbosieve SIII with... Hydrocarbons Carbosieve SIII with Alcohols Carbosieve SIII is a carbon based adsorbent resin. It is available from Supelco. Carbosieve SIII Breakthrough Volume Data Carbosieve SIII with Hydrocarbons Carbosieve SIII with Alcohols
Carbosieve SIII Breakthrough Volume Data Carbosieve SIII with... Hydrocarbons Carbosieve SIII with Alcohols Carbosieve SIII is a carbon based adsorbent resin. It is available from Supelco. Carbosieve SIII Breakthrough Volume Data Carbosieve SIII with Hydrocarbons Carbosieve SIII with Alcohols Glass Beads Breakthrough Volume Data Glass Beans with... Hydrocarbons Alcohols Glass beads are available from several suppliers. Glass Beads Breakthrough Volume Data Glass Beads with Hydrocarbons Glass Beads with Alcohols
Glass Beads Breakthrough Volume Data Glass Beans with... Hydrocarbons Alcohols Glass beads are available from several suppliers. Glass Beads Breakthrough Volume Data Glass Beads with Hydrocarbons Glass Beads with Alcohols
Breakthrough Volume Data By Organic Compounds
 Hydrocarbon Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of hydrocarbons via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain hydrocarbon (i.e. methane = 1, decane = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain hydrocarbon. The bars in the chart indicate the useful range of hydrocarbons that can be analyzed with the resin. The different...
Hydrocarbon Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of hydrocarbons via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain hydrocarbon (i.e. methane = 1, decane = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain hydrocarbon. The bars in the chart indicate the useful range of hydrocarbons that can be analyzed with the resin. The different...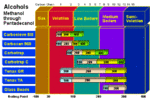 Alcohol Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of alcohols via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain alcohol (i.e. methanol = 1, decanol = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain alcohol. The bars in the chart indicate the useful range of alcohols that can be analyzed with the resin. The different colored bars ...
Alcohol Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of alcohols via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain alcohol (i.e. methanol = 1, decanol = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain alcohol. The bars in the chart indicate the useful range of alcohols that can be analyzed with the resin. The different colored bars ... Alkene Breakthrough Volumes for Adsorbent Resins Alkenes with ... Tenax® TA Tenax® TA with Alkenes Tenax® is a registered trademark of Buchem BV.
Alkene Breakthrough Volumes for Adsorbent Resins Alkenes with ... Tenax® TA Tenax® TA with Alkenes Tenax® is a registered trademark of Buchem BV. Acetate and Acid Breakthrough Volumes for Adsorbent Resins Acetates and Acids with ... Tenax® TA Tenax® TA with Acetates and Acids Tenax® is a registered trademark of Buchem BV.
Acetate and Acid Breakthrough Volumes for Adsorbent Resins Acetates and Acids with ... Tenax® TA Tenax® TA with Acetates and Acids Tenax® is a registered trademark of Buchem BV. Acetate and Acid Breakthrough Volumes for Adsorbent Resins Acetates and Acids with ... Tenax® TA Tenax® TA with Acetates and Acids Tenax® is a registered trademark of Buchem BV.
Acetate and Acid Breakthrough Volumes for Adsorbent Resins Acetates and Acids with ... Tenax® TA Tenax® TA with Acetates and Acids Tenax® is a registered trademark of Buchem BV. Aldehyde and Ketone Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of aldehydes via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain aldehyde (i.e. formaldehyde = 1, Decanal = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain aldehyde. The bars in the chart indicate the useful range of aldehyde that can be analyzed with the resin. The different colore...
Aldehyde and Ketone Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of aldehydes via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain aldehyde (i.e. formaldehyde = 1, Decanal = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain aldehyde. The bars in the chart indicate the useful range of aldehyde that can be analyzed with the resin. The different colore... Aldehyde and Ketone Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of aldehydes via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain aldehyde (i.e. formaldehyde = 1, Decanal = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain aldehyde. The bars in the chart indicate the useful range of aldehyde that can be analyzed with the resin. The different colore...
Aldehyde and Ketone Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of aldehydes via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain aldehyde (i.e. formaldehyde = 1, Decanal = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain aldehyde. The bars in the chart indicate the useful range of aldehyde that can be analyzed with the resin. The different colore... Halogen Breakthrough Volumes for Adsorbent Resins Halogens with ... Tenax® TA Tenax® GR Carboxen 569 Carbotrap Carbotrap C Tenax® TA with Halogens Tenax® GR with Halogens Carboxen 569 with Halogens Carbotrap with Halogens Carbotrap C with Halogens Tenax® is a registered trademark of Buchem BV.
Halogen Breakthrough Volumes for Adsorbent Resins Halogens with ... Tenax® TA Tenax® GR Carboxen 569 Carbotrap Carbotrap C Tenax® TA with Halogens Tenax® GR with Halogens Carboxen 569 with Halogens Carbotrap with Halogens Carbotrap C with Halogens Tenax® is a registered trademark of Buchem BV.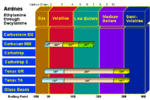 Amine Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of amines via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain amine (i.e. Ethylamine = 2, decylamine = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain aldehydes. The bars in the chart indicate the useful range of amines that can be analyzed with the resin. The different colored bars...
Amine Breakthrough Volumes for Adsorbent Resins The above chart demonstrates the useful range of the adsorbent resins for a range of amines via purge and trap thermal desorption techniques. The numbers at the top of the chart indicate the number of carbons in straight chain amine (i.e. Ethylamine = 2, decylamine = 10). The numbers at the bottom of the chart indicate the boiling point range of the straight chain aldehydes. The bars in the chart indicate the useful range of amines that can be analyzed with the resin. The different colored bars... Aromatics and Terpenes Breakthrough Volumes for Adsorbent
Resins Aromatics and Terpenes with ... Tenax® TA Tenax® GR Carboxen 569 Tenax® TA with Aromatics and Terpenes Tenax® GR with Aromatics and Terpenes Carboxen 569 with Aromatics and Terpenes Tenax® is a registered trademark of Buchem BV.
Aromatics and Terpenes Breakthrough Volumes for Adsorbent
Resins Aromatics and Terpenes with ... Tenax® TA Tenax® GR Carboxen 569 Tenax® TA with Aromatics and Terpenes Tenax® GR with Aromatics and Terpenes Carboxen 569 with Aromatics and Terpenes Tenax® is a registered trademark of Buchem BV.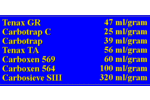 Water Breakthrough Volumes for Adsorbent Resins Water with ... Tenax® TA Breakthrough Volumes for Water at 20 degrees C for Various Resins The above chart indicates the Breakthrough Volumes for the various resins at 20 degrees C. The values are expressed as milliliters of gas per gram of adsorbent resin. The values expressed above are based on the manufacturers listed data with the exception of Tenax TA which we verified experimentally. It is important that the values above be doubled to assure complete elution of the water from the Adsorbe...
Water Breakthrough Volumes for Adsorbent Resins Water with ... Tenax® TA Breakthrough Volumes for Water at 20 degrees C for Various Resins The above chart indicates the Breakthrough Volumes for the various resins at 20 degrees C. The values are expressed as milliliters of gas per gram of adsorbent resin. The values expressed above are based on the manufacturers listed data with the exception of Tenax TA which we verified experimentally. It is important that the values above be doubled to assure complete elution of the water from the Adsorbe...
Backpressure Data
 Tenax® TA Back Pressure Versus Flow Data Tenax® TA is a porous polymer resin based on 2,6-diphenylene-oxide.
Tenax® TA Back Pressure Versus Flow Data Tenax® TA is a porous polymer resin based on 2,6-diphenylene-oxide. Tenax® GR Back Pressure Versus Flow Data Tenax® GR is a porous polymer resin based on 2,6-diphenylene-oxide plus 30% Graphite.
Tenax® GR Back Pressure Versus Flow Data Tenax® GR is a porous polymer resin based on 2,6-diphenylene-oxide plus 30% Graphite. Carbotrap and Carbotrap C Back Pressure Versus Flow Data Both Carbotrap and Carbotrap C are carbon based resins available from Supelco. Determination of Back Pressure Versus Flow for Adsorbent Resins A desorption tube filled with adsorbent resin can produce significant back pressures for the sampling pump when collecting a sample. If the back pressure is too high the air sampling pump will fail. For efficient operation the Back Pressure should not exceed 25. inches of water. In order to determine the Back Pressure at the end of the thermal desorption...
Carbotrap and Carbotrap C Back Pressure Versus Flow Data Both Carbotrap and Carbotrap C are carbon based resins available from Supelco. Determination of Back Pressure Versus Flow for Adsorbent Resins A desorption tube filled with adsorbent resin can produce significant back pressures for the sampling pump when collecting a sample. If the back pressure is too high the air sampling pump will fail. For efficient operation the Back Pressure should not exceed 25. inches of water. In order to determine the Back Pressure at the end of the thermal desorption... Carbotrap and Carbotrap C Back Pressure Versus Flow Data Both Carbotrap and Carbotrap C are carbon based resins available from Supelco. Determination of Back Pressure Versus Flow for Adsorbent Resins A desorption tube filled with adsorbent resin can produce significant back pressures for the sampling pump when collecting a sample. If the back pressure is too high the air sampling pump will fail. For efficient operation the Back Pressure should not exceed 25. inches of water. In order to determine the Back Pressure at the end of the thermal desorption...
Carbotrap and Carbotrap C Back Pressure Versus Flow Data Both Carbotrap and Carbotrap C are carbon based resins available from Supelco. Determination of Back Pressure Versus Flow for Adsorbent Resins A desorption tube filled with adsorbent resin can produce significant back pressures for the sampling pump when collecting a sample. If the back pressure is too high the air sampling pump will fail. For efficient operation the Back Pressure should not exceed 25. inches of water. In order to determine the Back Pressure at the end of the thermal desorption... Carbosieve SIII Back Pressure Versus Flow Data Carbosieve SIII is a carbon based adsorbent resin available from Supelco. Determination of Back Pressure Versus Flow for Adsorbent Resins A desorption tube filled with adsorbent resin can produce significant back pressures for the sampling pump when collecting a sample. If the back pressure is too high the air sampling pump will fail. For efficient operation the Back Pressure should not exceed 25. inches of water. In order to determine the Back Pressure at the end of the thermal desorption tube...
Carbosieve SIII Back Pressure Versus Flow Data Carbosieve SIII is a carbon based adsorbent resin available from Supelco. Determination of Back Pressure Versus Flow for Adsorbent Resins A desorption tube filled with adsorbent resin can produce significant back pressures for the sampling pump when collecting a sample. If the back pressure is too high the air sampling pump will fail. For efficient operation the Back Pressure should not exceed 25. inches of water. In order to determine the Back Pressure at the end of the thermal desorption tube...

