- ▶
- Heaters/Source
- ▶
- Agilent Heaters and SensorsMass Spectrometry, Scientific Supplies & ManufacturingScientific Instrument Services 5973 Source Heater Tamper Resistant Allen Wrench 5973/5975 Quad Sensor 5985 Source Heater Assembly Agilent Interface Heater Assembly 5971 Interface Heater

- ▶
- Reference Material on InstrumentationArticle - A High Temperature Direct Probe for a Mass Spectrometer Design of a Direct Exposure Probe and Controller for use ona Hewlett-Packard 5989 Mass Spectrometer SIS AP1000 AutoProbe™ SIS AP2000 AutoProbe™ - Description of System HPP7: Direct Probe Electronics Console HPP7: Direct Probe for the Agilent (HP) 5973/5975 MSD HPP7: HP Direct Probe Application Notes HPP7: Installation Directions for the Direct Probe HPP7: Side Cover for the HP 5973 MSD HPP7: Support HPP7: Probe Inlet System for the Agilent (HP) 5973 and 5975 MSD with Automatic Indexed Stops HPP7: Theory of Operation of the Direct Probe and Probe Inlet System Direct Thermal Extraction Thermal Desorption Application Notes Environmental Thermal Desorption Application Notes Food Science Thermal Desorption Application Notes Forensic Thermal Desorption Application Notes GC Cryo-Trap Application Notes Headspace Application Notes Purge & Trap Thermal Desorption Application Notes Theory of Operation of the AutoDesorb® System AutoDesorb Notes for SIS Dealers Adsorbent Resin Application Notes Installation of the Short Path Thermal Desorption System on Agilent (HP) and Other GCs Installation of the Short Path Thermal Desorption System on a Varian 3400 GC AutoDesorb® System Development Team Thermal Desorption Applications and Reference Materials Installation of the Short Path Thermal Desorption System - TD5 Part I - Design & Operation of the Short Path ThermalDesorption System Installation Instructions for the Model 951 GC Cryo-Trap on the HP 5890 Series GC Installation Instructions for the Model 961 GC Cryo-Trap on the HP 5890 Series GC Operation of the Model 951/961 GC Cryo-Trap SIS GC Cryo Traps - Theory of Operation NIST/EPA/NIH Mass Spectral Enhancements - 1998 version (NIST98) SIMION 3D Ion Optics Class Mass Spectrometer Source Cleaning Methods MS Tip: Mass Spectrometer Source Cleaning Procedures Mass Spec Source Cleaning Procedures Micro-Mesh® Abrasive Sheets Research Papers Using New Era Syringe Pump Systems EI Positive Ion Spectra for Perfluorokerosene (PFK) Cap Liner Information How do I convert between fluid oz and milliliters? Which bottle material should I choose? Which bottle mouth should I choose? The Bottle Selection Guide CGA Connections for Gas Tanks Chemical Reaction Interface Mass Spectrometry (CRIMS)

- LiteratureApplication Notes Adsorbent Resins Guide Mass Spec Tips SDS Sheets FAQ MS Calibration Compound Spectra Manuals MS Links/Labs/ Organizations MS Online Tools Flyers on Products/Services Scientific Supplies Catalog About Us NextAdvance Bullet Blender® Homogenizer Protocols Micro-Mesh® Literature Instrumentation Literature Agilent GC/MS Literature SIS News / E-Mail Newsletter NIST MS Database - Update Notifications

- ▶
- Chemical Reaction Interface Mass Spectrometry (CRIMS)51. Development and Characterization of a New Chemical Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) (EAS 96) 67. Using Chemical Reaction Interface (CRIMS) to Monitor Bacteria Transport in Situ (PittCon 98) 68. Using a Plug In UV-Vis Spectrometer to Monitor the Plasma Conditions in a GC CRIMS (EAS 98) 76. Determination of the Sensitivity of a CRIMS System (ASMS 98)

- Application NotesNote 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 96: Reducing Warping in Mass Spectrometer Filaments, with SISAlloy® Yttria/Rhenium Filaments Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 92: Yttria Coated Mass Spectrometer Filaments Note 91: AutoProbe DEP Probe Tip Temperatures Note 90: An Automated MS Direct Probe for use in an Open Access Environment Note 89: Quantitation of Organics via a Mass Spectrometer Automated Direct Probe Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 87: Design and Development of an Automated Direct Probe for a Mass Spectrometer Note 86: Simulation of a Unique Cylindrical Quadrupole Mass Analyzer Using SIMION 7.0. Note 85: Replacing an Electron Multiplier in the Agilent (HP) 5973 MSD Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 81: Rapid Bacterial Chemotaxonomy By DirectProbe/MSD Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 78: A New Solution to Eliminate MS Down-Time With No-Tool-Changing of Analytical GC Columns Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 76: Determination of the Sensitivity of a CRIMS System Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 72: 1998 Version of the NIST/EPA/NIH Mass Spectral Library, NIST98 Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 70: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part II Note 69: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part 1 Note 68: Use of a PC Plug-In UV-Vis Spectrometer To Monitor the Plasma Conditions In GC-CRIMS Note 67: Using Chemical Reaction Interface Mass Spectrometry (CRIMS) To Monitor Bacterial Transport In In Situ Bioremediation Note 66: Probe Tip Design For the Optimization of Direct Insertion Probe Performance Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization Note 61: Analysis of Sugars Via a New DEP Probe Tip For Use With theDirect Probe On the HP5973 MSD Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 59: Computer Modeling of a TOF Reflectron With Gridless Reflector Using SIMION 3D Note 58: Direct Probe Analysis and Identification of Multicomponent Pharmaceutical Samples via Electron Impact MS Note 57: Aroma Profiles of Lavandula species Note 56: Mass Spec Maintenance & Cleaning Utilizing Micro-Mesh® Abrasive Sheets Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 53: SIMION 3D v6.0 Ion Optics Simulation Software Note 52: Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry Using SIMION 3D Note 51: Development and Characterization of a New Chemical Reaction Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) Note 50: The Analysis of Multiple Component Drug Samples Using a Direct Probe Interfaced to the HP 5973 MSD Note 49: Analysis of Cocaine Utilizing a New Direct Insertion Probe on a Hewlett Packard 5973 MSD Note 48: Demonstration of Sensitivity Levels For the Detection of Caffeine Using a New Direct Probe and Inlet for the HP 5973 MSD Note 47: The Application Of SIMION 6.0 To Problems In Time-of-Flight Mass Spectrometry Note 46: Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond Note 45: Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources Note 44: The Design Of a New Direct Probe Inlet For a Mass Spectrometer Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS Tech No. "A" Note 14: Elimination of "Memory" Peaks in Thermal Desorption Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers - Part I of II Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers- Part II of II Adsorbent Resins Guide Development and Field Tests of an Automated Pyrolysis Insert for Gas Chromatography. Hydrocarbon Production in Pine by Direct Thermal Extraction A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Capillary (019P) - Comparison of Septa by Direct Thermal Extraction Volatile Organic Composition in Blueberry Identification of Volatile Organic Compounds in Office Products Detection and Indentification of Volatiles in Oil Base Paintsby Headspace GC with On Column Cryo-Trapping Evaluation of Septa Using a Direct Thermal Extraction Technique INFLUENCE OF STORAGE ON BLUEBERRY VOLATILES Selection of Thermal Desorption and Cryo-Trap Parameters in the Analysis of Teas Redesign and Performance of a Diffusion Based Solvent Removal Interface for LC/MS The Design of a New Direct Probe Inlet for a Mass Spectrometer Analytes Using Mass Spectrometry (CRIMS) Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources A Student Guide for SIMION Modeling Software Application of SIMION 6.0 to Problems in Time-of-flight Mass Spectrometry Comparison of Sensitivity of Headspace GC, Purge and TrapThermal Desorption and Direct Thermal Extraction Techniques forVolatile Organics The Influence of Pump Oil Purity on Roughing Pumps Analysis of Motor Oils Using Thermal Desorption-Gas Chromatography-Mass Spectrometry IDENTIFICATION OF VOLATILE ORGANIC COMPOUNDS IN PAPER PRODUCTS Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry using SIMION 3D Seasonal Variation in Flower Volatiles Development of and Automated Microprocessor Controlled Gas chromatograph Fraction Collector / Olfactometer Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Column Design of a Microprocessor Controlled Short Path Thermal Desorption Autosampler Computer Modeling of Ion Optics in Time-of-Flight Mass Spectrometry Using SIMION 3D Thermal Desorption Instrumentation for Characterization of Odors and Flavors

- ▶
- 51. Development and Characterization of a New Chemical Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) (EAS 96) (This Page)
Jiuwei Teng, Yohannes Teffera, and Fred Abramson, Dept. of Pharmacology, The George Washington University, 2300 Eye Street, NW, Washington, DC 20037.
(presented at EAS '96)
INTRODUTION
We report the development of an improved microwave cavity for use in Chemical Reaction Interface Mass Spectrometry (CRIMS). CRIMS relies on mass ratio measurement for the identification of isotopically labeled species. The accurate measurement of isotope ratios eliminates the need for radioactive species as tracers. This approach has many advantages and applications particularly in the study of pharmocological agents.
Background
The CRIMS method was developed at George Washington University in the early 1980's [1-3]. The technique replaces the need for radioactive tracers by measuring the ratios of stable isotopes. In order to distinguish isotopes of different mass from molecular species, as (M+H)+ etc., the analytes are broken down and mixed with a reactant gas in a microwave cavity. This destroys chemical information about the original molecule but generates the same simple products for all precursors. For example, analytes containing enriched 13C may be detected by reaction with SO2 to produce CO2. The ratio of 12CO2 (44 amu) and 13CO2 (45 amu) may then be monitored to selectively identify 13C enriched analytes.
CRIMS
Chemistry
Abramson [3] and others [4-6] have developed a variety of CRIMS chemistries to distinguish between the isotopes of specific elements. These elements include: H, C, N, O, P, S, Cl, Se, and Br. The reactant gases employed include NF3, SO2, H2, N2, and HCl of which NF3 and SO2 appear to be the most versatile. Key to the selection of reactant gas is the generation of volatile and stable products whose masses do not overlap with other atomic or molecular species. It is preferable to generate products, as HF and DF, which have unique masses in comparison to products, as 1H2H, which requires high mass resolution to be distinguished from 1H3+. Signals resulting from the natural abundance of the tracer isotope can easily be subtracted from the total signal to determine that part resulting solely from enrichment. For example, enriched 13CO2 signal can be found from 13CO2 = m/z 45 - 0.0119 * m/z 44. The factor 0.0119 accounts for both the natural 13C and 17O contributions.

Figure 1 - CRIMS Cavity
CRIMS Advantages
CRIMS is intended to eliminate the need for radioactive tracers by determining the ratios of stable isotopes. Radioactive tracers are currently used in a variety of industries and have the advantage that other than a small change in mass the tagged species is not chemically modified. Their primary disadvantages are the need to produce and handle radioactive material and the dangers of introducing radioactive isotopes into living organisms. This is a severe limitation in Pharmacology and Biochemistry. Other techniques that can distinguish stable isotopes include inductively coupled plasma mass spectrometry, inductively coupled and microwave-induced plasma spectroscopy, and isotope-ratio monitoring mass spectrometry. The last of these techniques is the closest analog of CRIMS. It involves the complete oxidation of the analyte in a CuO furnace and the mass spectrometric detection of CO2 from which carbon isotopic composition can be determined. Matthews and Hayes [7] report performance for a CuO furnace similar to that of the chemical reaction interface. CRIMS has the advantages that it operates at lower pressure so that the volume of the reaction cavity has a much smaller effect on chromatographic band broadening. The CuO furnace also requires regular replacement or regeneration of the CuO. In contrast, the low pressure of the CRIMS cavity means that the consumption on reactant gas is minimal. An additional advantage of the CRIMS system is that 13C and 15N can be monitored simultaneously whereas the furnace chemistry requires that CO2 be removed from the system to avoid interference between N2 and CO. While radiochemical detection methods are normally considered highly sensitive, this is only true for off-line measurements where analysis time is not a limiting factor. In on-line measurements, as those made when analyzing chromatographic effluent, the detection time is limited to the width of the chromatographic peak and the detection limits are dramatically reduced. As a consequence, both GC/CRIMS and HPLC/CRIMS have been shown to produce substantially better signal to noise and/or resolution then on-line radiometric detection (for 14C) [3].
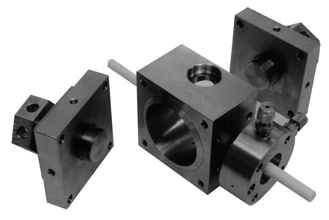
Figure 2 - Dissambled CRIMS cavity
Design of a CRIMS Cavity
A new CRIMS cavity is shown in Figure 1. This device is either placed inside a GC oven for GC/CRIMS or after a desolvation apparatus for HPLC/CRIMS. In either case, a transfer line (fused silica capillary) is used to transport the reaction products to a mass spectrometer. Microwave power is supplied through a gold plated sidearm (long enough to reach outside of a GC oven if needed.) The cavity is tuned via two finely threaded pole pieces located on opposite ends of the cavity. These parts are indicated in the disassembled cavity shown in Figure 2. Also shown are the reactant gas inlet and reaction tube. The (1/16 mm i.d.) reaction tube confines the chromatographic effluent and maintains a low pressure to sustain a plasma. The plasma occurs only inside of this tube since the remainder of the cavity is at atmospheric pressure. A number of improvements have been made in this version of the microwave cavity. These addressed problems that had been observed in earlier versions. First among these was poor electrical conductivity between the pole pieces and the bulk of the cavity. This contact is made along a 3/4-32 thread. When the pole piece was rotated, intermittent changes in impedance affected the tuning of the cavity. A second problem was the deterioration of the center conductor in the side arm. This piece (12 gauge Cu rod) become very hot when the cavity was in use and could be bent in the process of connecting a cable. Contact between the pole pieces and the CRIMS body was improved by the introduction of gold gaskets and a compression mechanism. This is shown in Figure 3A. It is now possible to tighten the end plates and assure good electrical contact between the body and poles. This is expected to dramatically improve the tuning process for the microwave plasma. Initial (DC) resistance measurements have been performed and these indicate that an improvement has been made. (See below.) Two changes have been made in the construction of the side arm in order to improve the lifetime of the center conductor. First, additional PTFE supports have been placed inside the arm to prevent bending of the rod when connectors are attached. This will prevent shorting between the center rod and outer tube. (An event that can damage the microwave power supply.) Second, the connection between the center rod and the induction loop has been made with a screw instead of a silver solder joint. The center rod can therefore be easily exchanged and the need to replace the entire sidearm is eliminated. A view of the side arm showing the induction loop and PTFE supports is presented in Figure 3B.
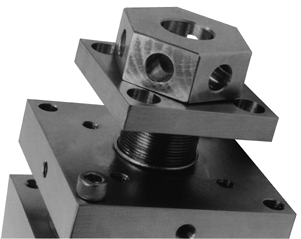
Figure 3A - CRIMS Gold Gasket Compression Mechanism
Measurements Measurements have been made of the resistance between the CRIMS body and pole pieces. This was accomplished with a Keithley 2010 multimeter and Kelvin (4 lead) probes. The results are shown in Figure 4. The relative standard deviation of the measurements improved from 90 to 44 % with the introduction of the gold gasket. The average resistance was also reduced from 15 to 0.36 milliohms. Between each measurement the pole piece was rotated and the gasket was tightened if present. While these measurements were made with DC potentials it is hoped that similar results will be observed at microwave frequencies.

Figure 3B - CRIMS Center Rod
Conclusions
A new microwave cavity for use in Chemical Reaction Interface Mass Spectrometry has been produced. This cavity includes several improvements over previous designs and is expected to be both more reliable and easier to use. The device is currently undergoing tests as part of a complete CRIMS system. It will be examined for plasma stability and component lifetime. Further results will be made available at: http://www.sisweb.com
References
1. Abramson F. P.; Markey, S. P. Proc ASMS Conf. 1982, 30, 866- 867.
2. Markey S. P.; Abramson F. P. Anal. Chem. 1982, 54, 2375-6.
3. Abramson F. P. Mass Spectrom. Rev. 1994, 13, 341-356.
4. Heppner, R. A. Anal. Chem. 1983, xx, 2170-2174.
5. Morre', J. T.; Moini, M. Biol. Mass Spectrom.1992, 21, 693-9.
6. Li, G.; Moini, M.; Rerez, F.; Ibarra, F. E.; Sandoval, D. Proc. ASMS Conf. 1994, 42, 293-4.
7. Matthews, D. E.; Hayes, J. M. Anal. Chem. 1978, 50, 1465-73.

