- ▶
- Heaters/Source
- ▶
- Agilent Heaters and SensorsMass Spectrometry, Scientific Supplies & ManufacturingScientific Instrument Services 5973 Source Heater Tamper Resistant Allen Wrench 5973/5975 Quad Sensor 5985 Source Heater Assembly Agilent Interface Heater Assembly 5971 Interface Heater

- ▶
- LiteratureApplication Notes Adsorbent Resins Guide Mass Spec Tips SDS Sheets FAQ MS Calibration Compound Spectra Manuals MS Links/Labs/ Organizations MS Online Tools Flyers on Products/Services Scientific Supplies Catalog About Us NextAdvance Bullet Blender® Homogenizer Protocols Micro-Mesh® Literature Instrumentation Literature Agilent GC/MS Literature SIS News / E-Mail Newsletter NIST MS Database - Update Notifications

- ▶
- Application NotesNote 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 96: Reducing Warping in Mass Spectrometer Filaments, with SISAlloy® Yttria/Rhenium Filaments Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 92: Yttria Coated Mass Spectrometer Filaments Note 91: AutoProbe DEP Probe Tip Temperatures Note 90: An Automated MS Direct Probe for use in an Open Access Environment Note 89: Quantitation of Organics via a Mass Spectrometer Automated Direct Probe Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 87: Design and Development of an Automated Direct Probe for a Mass Spectrometer Note 86: Simulation of a Unique Cylindrical Quadrupole Mass Analyzer Using SIMION 7.0. Note 85: Replacing an Electron Multiplier in the Agilent (HP) 5973 MSD Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 81: Rapid Bacterial Chemotaxonomy By DirectProbe/MSD Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 78: A New Solution to Eliminate MS Down-Time With No-Tool-Changing of Analytical GC Columns Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 76: Determination of the Sensitivity of a CRIMS System Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 72: 1998 Version of the NIST/EPA/NIH Mass Spectral Library, NIST98 Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 70: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part II Note 69: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part 1 Note 68: Use of a PC Plug-In UV-Vis Spectrometer To Monitor the Plasma Conditions In GC-CRIMS Note 67: Using Chemical Reaction Interface Mass Spectrometry (CRIMS) To Monitor Bacterial Transport In In Situ Bioremediation Note 66: Probe Tip Design For the Optimization of Direct Insertion Probe Performance Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization Note 61: Analysis of Sugars Via a New DEP Probe Tip For Use With theDirect Probe On the HP5973 MSD Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 59: Computer Modeling of a TOF Reflectron With Gridless Reflector Using SIMION 3D Note 58: Direct Probe Analysis and Identification of Multicomponent Pharmaceutical Samples via Electron Impact MS Note 57: Aroma Profiles of Lavandula species Note 56: Mass Spec Maintenance & Cleaning Utilizing Micro-Mesh® Abrasive Sheets Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 53: SIMION 3D v6.0 Ion Optics Simulation Software Note 52: Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry Using SIMION 3D Note 51: Development and Characterization of a New Chemical Reaction Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) Note 50: The Analysis of Multiple Component Drug Samples Using a Direct Probe Interfaced to the HP 5973 MSD Note 49: Analysis of Cocaine Utilizing a New Direct Insertion Probe on a Hewlett Packard 5973 MSD Note 48: Demonstration of Sensitivity Levels For the Detection of Caffeine Using a New Direct Probe and Inlet for the HP 5973 MSD Note 47: The Application Of SIMION 6.0 To Problems In Time-of-Flight Mass Spectrometry Note 46: Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond Note 45: Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources Note 44: The Design Of a New Direct Probe Inlet For a Mass Spectrometer Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS Tech No. "A" Note 14: Elimination of "Memory" Peaks in Thermal Desorption Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers - Part I of II Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers- Part II of II Adsorbent Resins Guide Development and Field Tests of an Automated Pyrolysis Insert for Gas Chromatography. Hydrocarbon Production in Pine by Direct Thermal Extraction A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Capillary (019P) - Comparison of Septa by Direct Thermal Extraction Volatile Organic Composition in Blueberry Identification of Volatile Organic Compounds in Office Products Detection and Indentification of Volatiles in Oil Base Paintsby Headspace GC with On Column Cryo-Trapping Evaluation of Septa Using a Direct Thermal Extraction Technique INFLUENCE OF STORAGE ON BLUEBERRY VOLATILES Selection of Thermal Desorption and Cryo-Trap Parameters in the Analysis of Teas Redesign and Performance of a Diffusion Based Solvent Removal Interface for LC/MS The Design of a New Direct Probe Inlet for a Mass Spectrometer Analytes Using Mass Spectrometry (CRIMS) Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources A Student Guide for SIMION Modeling Software Application of SIMION 6.0 to Problems in Time-of-flight Mass Spectrometry Comparison of Sensitivity of Headspace GC, Purge and TrapThermal Desorption and Direct Thermal Extraction Techniques forVolatile Organics The Influence of Pump Oil Purity on Roughing Pumps Analysis of Motor Oils Using Thermal Desorption-Gas Chromatography-Mass Spectrometry IDENTIFICATION OF VOLATILE ORGANIC COMPOUNDS IN PAPER PRODUCTS Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry using SIMION 3D Seasonal Variation in Flower Volatiles Development of and Automated Microprocessor Controlled Gas chromatograph Fraction Collector / Olfactometer Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Column Design of a Microprocessor Controlled Short Path Thermal Desorption Autosampler Computer Modeling of Ion Optics in Time-of-Flight Mass Spectrometry Using SIMION 3D Thermal Desorption Instrumentation for Characterization of Odors and Flavors

- ▶
- Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D (This Page)
Presented at EAS, Summerset, NJ., November 1998
INTRODUCTION
The propose of this poster is to demonstrate that methods used to improve the resolution of MALDI-TOF instruments can be extended to other TOF instruments. Specifically, we examine the calculation of instrument conditions in an electrospray-TOF instrument. Instrument performance is simulated using SIMION 3D ion optics software.
A review is given of the methods for calculating instrument conditions in time of flight instruments. This includes: 1. the calculation of expected time of flight; 2. the calculation of space focusing conditions; and 3. the calculation of improved conditions for MALDI instruments. We then demonstrate how similar calculations can be made to determine improved conditions for an electrospray-TOF geometry. Finally, simulated results are shown and discussed.
Calculation of Ion Time of Flight
The flight time of an ion can be calculated from the following fundamental equations:
![]()
Where Ax is the acceleration in region x in units of meters/sec2, FC is the Faraday Constant, Ex is the electric field in region x (Volts/meter), and mass is the ion's mass in Daltons.
Velocity (Vx) at the end of an acceleration over a distance of length Dx is given by
![]()
Where Vx-1 is the velocity prior to the effects of Ax.
The time (Tx) spent in an acceleration region is given by
![]()
The time spent in a field free region is given by Tx = Dx/Vx and the total time of flight (Ttotal )is the sum of the times spent in each region.
![]()
Calculation of Space Focusing Conditions
Space focusing conditions are intended to minimize the dependence of Ttotal on the initial ion position. This position is typically referred to as X0. In the above notation X0 can be defined relative to the end of the first acceleration region and thus X0 = D1.
In order to find space focusing conditions, as defined
by Wiley and McLaren (1), one first assumes that the average initial velocity
(V0) is equal to zero. The derivative ![]() is
then set equal to zero and one can solve for a ratio of the first two acceleration
regions (E1/E2). (In a linear instrument with a two
step acceleration source followed by a drift region). By selecting a drift
energy in the field free region, the voltages to be applied in the source
can then be determined. Note that for instrument geometries where the average
initial velocity is not equal to zero, as MALDI-TOF instruments, space
focusing calculations as describe by Wiley and McLaren do not provide the
optimal answer.
is
then set equal to zero and one can solve for a ratio of the first two acceleration
regions (E1/E2). (In a linear instrument with a two
step acceleration source followed by a drift region). By selecting a drift
energy in the field free region, the voltages to be applied in the source
can then be determined. Note that for instrument geometries where the average
initial velocity is not equal to zero, as MALDI-TOF instruments, space
focusing calculations as describe by Wiley and McLaren do not provide the
optimal answer.
Calculation of Focusing Conditions In a MALDI-TOF
In a MALDI-TOF instrument, where ions are desorbed towards the detector, the average initial velocity of ions does not equal zero. There are two approaches that may be taken to avoid the assumptions made by Wiley and McLaren (2,3). First, one could assume an average non-zero initial velocity. This has the drawback that initial velocities are known to vary widely. Thus, any single value would be a poor representative for all the ions. In an alternative approach one could use the relationship V0 = (D-X0)/t. Where D is the total length of the first acceleration region and t is a delay time between the desorption of the analytes and their acceleration toward the detector. This equation differs from that used by Wiley and McLaren, in their description of time lag focusing, in that it expresses a relationship between the velocity of an ion and its distance from the sample plate. In contrast, time-lag-focusing utilizes an equation that relates an ion's velocity with its displacement from an unknown origin. The above equation can, therefore, be used to correlate the spatial and velocity distributions at the time of extraction. By substituting it into the basic equation for time of flight discussed above, the V0 term can be eliminated. X0 then represents both the velocity and spatial distributions at the time of extraction. Optimal focusing conditions can now be found by taking the derivative to the time of flight with respect to X0. No further assumptions about V0 need to be made. In practice, other optimization routines (i.e. SIMPLEX optimization) are preferable since under the best conditions the time of flight as a function of X0 is no longer a simple parabola (2). Alternative optimization routines are simplified by the elimination of the independent V0 variable. They also permit one to optimize other parameters such as the delay time before acceleration.
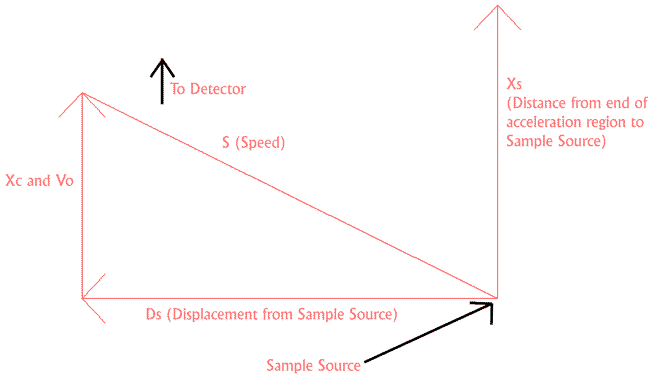
Figure 1. Definition of Instrument Source Parameters
The above calculations have dramatically improved the performance of MALDI instruments since they were first introduced in 1994 (4,5,6). The same principles can be applied to other instrument geometries (3). These include systems in which the sample is desorbed from a surface parallel to the main axis of the flight tube and systems in which sample is continuously introduced in a direction perpendicular to the main axis of the flight tube (See Figure 1). This second geometry may be relevant to some electrospray-TOF systems. The remainder of this poster examines how improved focusing conditions may be found for systems with an electrospray type geometry. SIMION 3D software is used to estimate improvements in peak width and instrument resolution.
The Application of Correlation Principles To Instruments With an "Electrospray" TOF Geometry
Figure 1 illustrates the relevant geometry and defines the distance (X, D) and velocity (V) variables used below. It is assumed that the sample continuously enters the instrument from a point referred to as the sample source. A voltage pulse is periodically applied to the first acceleration region to extract ions toward the detector. The correlation equation for this geometry is not as simple as the equation used in the MALDI geometry. For this geometry
![]()
Where X0 = Xs - Xc. Xs represents the displacement between the sample source and the end of the first acceleration region. Ds represents the displacement, perpendicular to Xs, between the sample source and the point of ionization. S represents the speed at which the sample leaves the sample source. We are assuming that S and Xs are the same for all molecules and we are only considering a two dimensional view of the source. We will also have to take into account the fact that that Ds will take a range of values. The above equations can be substituted into a basic equation for the time of flight of an ion. The resulting formula can then be used to calculate optimal instrument conditions.
Calculation of Optimal Instrument Conditions For the Electrospray-TOF Instrument
For the purposes of this poster we have calculated optimal conditions using an Excel spreadsheet. We assumed the patameters listed in Table 1. These conditions are intentionally different from any commercial instrument of which we are aware. Our purpose is to demonstrate calculation and simulation methods, not to find improved focusing conditions for any specific instrument.
Table 1
| Ds | 3 ± 0.2 cm | Xs | 5 mm |
| D1 | 1 cm | D2 | 8 mm |
| D3 (Field Free) | 80 cm | Drift Energy | 5000 eV |
| Ion Mass/Charge | 5,000 | Ion Speed | 982 m/s |
Optimal values were found for the two non-zero voltages in the instrument source. It was assumed that ions leaving the sample source at angles of greater than six degrees did not reach the extraction region. A calculation was also performed using the space focusing methods of Wiley and McLaren. These results were used for comparison. The results obtained are shown in Table 2 below.
Table 2
| Calculation | Variable | Value (Volts) |
| Correlation | First Voltage | 5284.0 |
| Second Voltage | 4216.0 | |
| Space Focusing | First Voltage | 5257.12 |
| Second Voltage | 4742.88 |
Simulation of Calculated Results
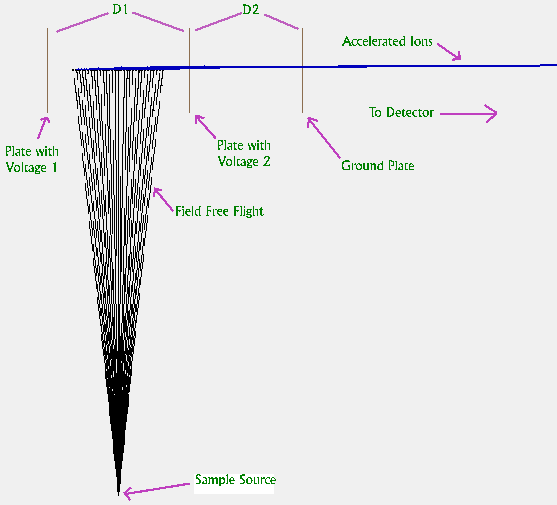
Figure 2. Instrument Simulation In SIMION 3D Software
In order to demonstrate the improvement in peak width resulting from correlation conditions, simulations were performed using the ion optics software SIMION 3D (7). An illustration from this simulation is shown in Figure 2. Ions first appear from below and travel to the center of the instrument source. A voltage pulse is then applied and ions are rapidly accelerated toward the detector (not shown). To clarify the illustration, we have changed the color of the ion trajectory at the time the voltage pulse is applied. In the figure 30 ions are shown; however, in the simulation, two thousand ions were flown. They were given initial angles between ± six degrees and were extracted at five possible values of Ds between 28 and 32 mm.
Results.
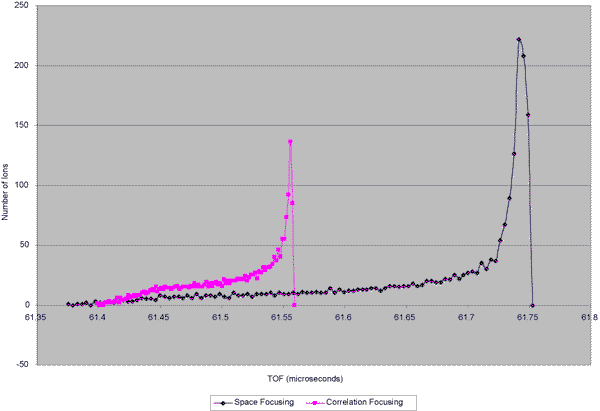
Figure 3 Calculated Results
Ion times of flight were recorded and histograms were calculated in order to generate possible peak shapes. The resulting plots are shown in Figure 3. The simulated peak resulting from correlation conditions is substantially narrower than that resulting from Space Focusing conditions. These results predict a narrowing of experimental peaks by about 60%. This improvement is not as dramatic as that found in the MALDI geometry do to the various possible values of Ds. However, they do show a potential improvement to instrument resolution.
Conclusions
We have demonstrated methods in which improved instrument conditions may be calculated for a specific geometry of TOF instrument. These conditions have been simulated using SIMION 3D software. We have made significant assumptions in our calculations. For example, we have used a very simple instrument without items such as focusing elements between the sample source and the instrument source. We have also not included space charge effects, we have assumed that Xs is the same for all analytes, and we have not accounted for changes in velocity due to collisions or the desolvation process. These factors may require changes to the correlation equation and may effect the improvement that is possible in a real instrument.
References
- Wiley, W. C.; McLaren I. H. Rev. Sci. Instrum. 1955, 26, 1150-1157.
- Colby, S. M.; Reilly, J. P. Anal. Chem. 1996, 68, 1419-1428.
- U.S. Patents 5,504,326; 5,510,613, and 5,712,479
- Brown, R. S.; Lennon, J. J.; Christie, D. Desorption '94, Sunriver Lodge, OR, March 27-31, 1994, p. 63. and Lennon, J. J.; Brown, R. S. 42nd ASMS Conf. On Mass Spectrom. 1994, 501.
- Colby, S. M.; King, T. B.; Reilly, J. P. Rapid Commun. Mass Spectrom. 1994, 8, 865-868.
- Whittal, R. M.; Li, L. Anal. Chem. 1995, 67, 1950-1954.
- SIMION 3D software by David Dahl. See www.sisweb.com.

