- ▶
- Heaters/Source
- ▶
- Agilent Heaters and SensorsMass Spectrometry, Scientific Supplies & ManufacturingScientific Instrument Services 5973 Source Heater Tamper Resistant Allen Wrench 5973/5975 Quad Sensor 5985 Source Heater Assembly Agilent Interface Heater Assembly 5971 Interface Heater

- ▶
- Probes
- LiteratureApplication Notes Adsorbent Resins Guide Mass Spec Tips SDS Sheets FAQ MS Calibration Compound Spectra Manuals MS Links/Labs/ Organizations MS Online Tools Flyers on Products/Services Scientific Supplies Catalog About Us NextAdvance Bullet Blender® Homogenizer Protocols Micro-Mesh® Literature Instrumentation Literature Agilent GC/MS Literature SIS News / E-Mail Newsletter NIST MS Database - Update Notifications

- ▶
- Application NotesNote 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 96: Reducing Warping in Mass Spectrometer Filaments, with SISAlloy® Yttria/Rhenium Filaments Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 92: Yttria Coated Mass Spectrometer Filaments Note 91: AutoProbe DEP Probe Tip Temperatures Note 90: An Automated MS Direct Probe for use in an Open Access Environment Note 89: Quantitation of Organics via a Mass Spectrometer Automated Direct Probe Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 87: Design and Development of an Automated Direct Probe for a Mass Spectrometer Note 86: Simulation of a Unique Cylindrical Quadrupole Mass Analyzer Using SIMION 7.0. Note 85: Replacing an Electron Multiplier in the Agilent (HP) 5973 MSD Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 81: Rapid Bacterial Chemotaxonomy By DirectProbe/MSD Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 78: A New Solution to Eliminate MS Down-Time With No-Tool-Changing of Analytical GC Columns Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 76: Determination of the Sensitivity of a CRIMS System Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 72: 1998 Version of the NIST/EPA/NIH Mass Spectral Library, NIST98 Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 70: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part II Note 69: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part 1 Note 68: Use of a PC Plug-In UV-Vis Spectrometer To Monitor the Plasma Conditions In GC-CRIMS Note 67: Using Chemical Reaction Interface Mass Spectrometry (CRIMS) To Monitor Bacterial Transport In In Situ Bioremediation Note 66: Probe Tip Design For the Optimization of Direct Insertion Probe Performance Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization Note 61: Analysis of Sugars Via a New DEP Probe Tip For Use With theDirect Probe On the HP5973 MSD Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 59: Computer Modeling of a TOF Reflectron With Gridless Reflector Using SIMION 3D Note 58: Direct Probe Analysis and Identification of Multicomponent Pharmaceutical Samples via Electron Impact MS Note 57: Aroma Profiles of Lavandula species Note 56: Mass Spec Maintenance & Cleaning Utilizing Micro-Mesh® Abrasive Sheets Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 53: SIMION 3D v6.0 Ion Optics Simulation Software Note 52: Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry Using SIMION 3D Note 51: Development and Characterization of a New Chemical Reaction Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) Note 50: The Analysis of Multiple Component Drug Samples Using a Direct Probe Interfaced to the HP 5973 MSD Note 49: Analysis of Cocaine Utilizing a New Direct Insertion Probe on a Hewlett Packard 5973 MSD Note 48: Demonstration of Sensitivity Levels For the Detection of Caffeine Using a New Direct Probe and Inlet for the HP 5973 MSD Note 47: The Application Of SIMION 6.0 To Problems In Time-of-Flight Mass Spectrometry Note 46: Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond Note 45: Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources Note 44: The Design Of a New Direct Probe Inlet For a Mass Spectrometer Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS Tech No. "A" Note 14: Elimination of "Memory" Peaks in Thermal Desorption Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers - Part I of II Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers- Part II of II Adsorbent Resins Guide Development and Field Tests of an Automated Pyrolysis Insert for Gas Chromatography. Hydrocarbon Production in Pine by Direct Thermal Extraction A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Capillary (019P) - Comparison of Septa by Direct Thermal Extraction Volatile Organic Composition in Blueberry Identification of Volatile Organic Compounds in Office Products Detection and Indentification of Volatiles in Oil Base Paintsby Headspace GC with On Column Cryo-Trapping Evaluation of Septa Using a Direct Thermal Extraction Technique INFLUENCE OF STORAGE ON BLUEBERRY VOLATILES Selection of Thermal Desorption and Cryo-Trap Parameters in the Analysis of Teas Redesign and Performance of a Diffusion Based Solvent Removal Interface for LC/MS The Design of a New Direct Probe Inlet for a Mass Spectrometer Analytes Using Mass Spectrometry (CRIMS) Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources A Student Guide for SIMION Modeling Software Application of SIMION 6.0 to Problems in Time-of-flight Mass Spectrometry Comparison of Sensitivity of Headspace GC, Purge and TrapThermal Desorption and Direct Thermal Extraction Techniques forVolatile Organics The Influence of Pump Oil Purity on Roughing Pumps Analysis of Motor Oils Using Thermal Desorption-Gas Chromatography-Mass Spectrometry IDENTIFICATION OF VOLATILE ORGANIC COMPOUNDS IN PAPER PRODUCTS Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry using SIMION 3D Seasonal Variation in Flower Volatiles Development of and Automated Microprocessor Controlled Gas chromatograph Fraction Collector / Olfactometer Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Column Design of a Microprocessor Controlled Short Path Thermal Desorption Autosampler Computer Modeling of Ion Optics in Time-of-Flight Mass Spectrometry Using SIMION 3D Thermal Desorption Instrumentation for Characterization of Odors and Flavors

- ▶
- Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization (This Page)
By Steve M. Colby
Presented at ASMS, Palm Springs, CA., June 1997
INTRODUCTION
Quality control and process monitoring in the production of polymers requires a quick and simple method of analysis. We demonstrate the analysis of polymers using a direct insertion probe and the 5973 MSD. Emphasis is placed on the ability to identify impurities and the correct monomer composition. Average analysis time is under 5 minutes including sample preparation.
The manufacture of polymers requires careful monitoring and product characterization. This is often done via chromatography and may include the pyrolysis of the product material. These analyses are difficult to perform during production because of the time required for chromatography. Impurities or other problems in manufacture may therefore not be discovered until after significant material and time has been lost. Analysis via direct insertion probe and EI mass spectrometry can be performed within 5 minutes of sample collection from the process stream. This allows for relatively quick monitoring of manufacturing conditions. We describe the use of a new high temperature probe compatible with a bench top mass spectrometer and demonstrate the mass spectral information that can be obtained.
Experimental
A new microprocessor and computer controlled direct probe is used with an HP 5973 MSD mass spectrometer. The probe is capable of heating to 450 oC at rates of over 1000 degrees per minute. The probe control system is interfaced directly with the HP ChemStation software. This increases the reproducibility of data, since the probe temperature program can be coordinated with the mass spectrometer's data acquisition. It also allows the probe temperature program to be saved with the data files for improved QC and GLP procedures. In addition, the probe can be programmed to ramp at rates between 1 and 500 degrees per minute. Up to 14 ramp steps can be defined. The probe software incorporates a "Hold" feature that allows the user to stop a temperature program for a indefinite period. This unique feature allows the user to respond to the data as it is being collected and optimize acquisition conditions during the analysis process. Figure 1 shows the main screen for the probe control program. It includes a graphic illustrating the entered temperature profile.
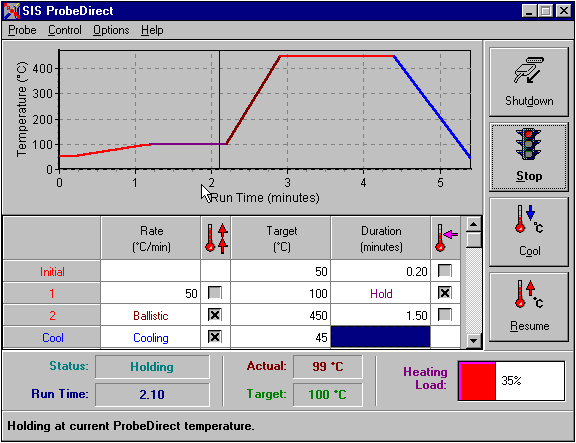
Figure 1. Probe Control Screen
Ethylene/Vinyl Acetate Copolymer samples were purchased from Scientific Polymer Products, Inc. Copolymers with compositions of 9, 28, 50, and 70 percent Vinyl Acetate were dissolved in Toluene to form solutions of 1ug/uL. 1 uL aliquots were placed in probe vials for use in each analysis. The vials were tapped so that most of the sample was collected at the bottom of the vials. Toluene was evaporated by placing the vials in a heating block at 70 oC. It should be noted that the polymers could have been analyzed directly without the use of a solvent. This, however, would have reduced our ability to introduce small quantitative samples into the mass spectrometer. Samples were ballistically heated in the mass spectrometer to 450 oC. Ballistic heating resulted in a heating rate of approximately 1000 degrees per minute. Mass spectra were recorded for 3.53 minutes. Although, the data below shows that all useful information was obtained in less than 2 minutes.
Results
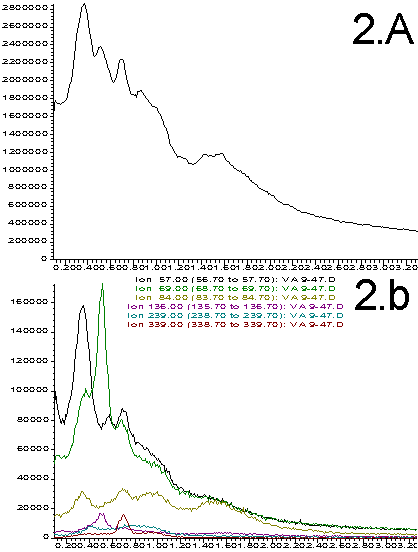
The total ion signal recorded for each of the four samples is shown in Figures 2A through 5A. Each sample produced a unique and reproducible profile. The reproducibility achieved is illustrated in Figure 6. This data includes 4 analyses of the 9% Vinyl Acetate sample. While there is variation in the total ion profile, it is always distinguishable from the signals obtained from the other samples. The variations may result from several factors.
The most critical aspect of reproducibility is the heating of the sample. The sample material may experience a range of temperatures as a function of its position within the sample vial. The top of the vial is influenced by the temperature of the mass spectrometer source and therefore can be at a different temperature than the bottom of the vial. If sample material is distributed at several location along the length of the vial, different parts of the analyte will experience different temperatures during the temperature program. This can even result in additional unwanted peaks. The heating of the probe tip is reproducible within 2 to 3 seconds, however, small variations in the thickness of the vial and the precise contact points between the glass and stainless steel of the probe can also effect the sample temperature significantly. We have found that using the same vial improves reproducibility. We are therefore investigating possible improvements in the vial manufacturing process.
 F
F
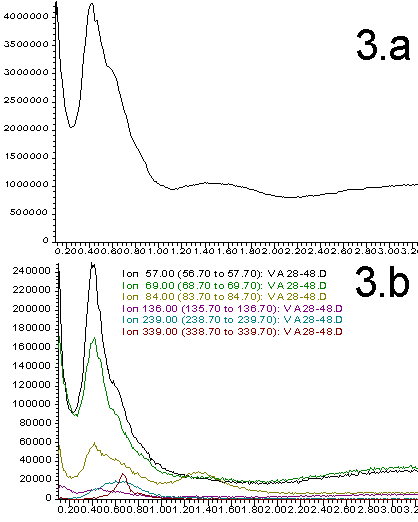
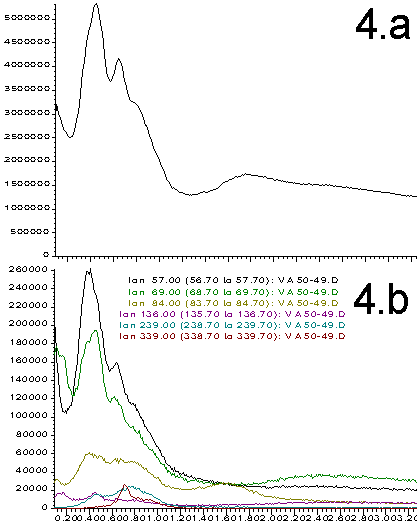
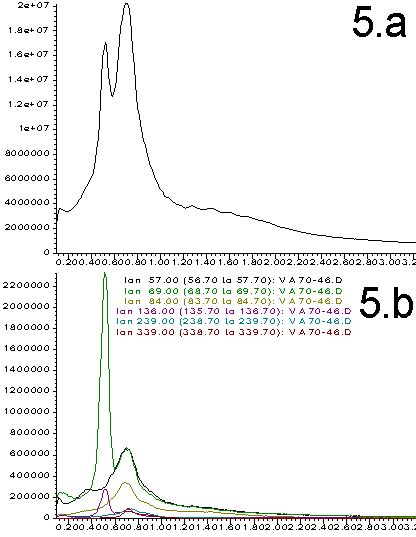
Figures 2-5. A. Total ion profiles; B. Extracted Ion Chromatograms (figures 2=9%; 3=28%, 4=50%, 5=70%)
The total ion profiles shown in Figures 2A through 5A clearly indicate that a variety of poorly resolved species are detected when the probe is heated. These species include analyte components as well as products resulting from the thermal degradation and pyrolysis of the sample. The exact identity and ratio of the thermal degradation products is a function of the original polymer composition. Prediction of the signal intensity involves a statistical analysis of original monomer ratios as well as the expected fragmentation patterns. We have not yet attempted these calculations. Instead, our purpose is to show that the original polymer composition can have a dramatic effects on the data obtained and that this data can be used as a quality control tool.
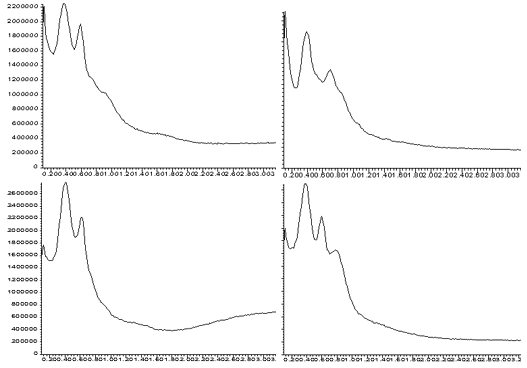
Figure 6. Reproducibility of Total Ion Profile For 9% Sample
More information can be extracted from the total ion profiles using the HP ChemStation data manipulation software. "Extracted ion chromatograms" and background subtraction enables us to identify specific masses that correlate to most of the components that contribute to the total ion profiles. Extracted ion chromatograms are shown in Figures 2B through 5B. The masses selected are chosen to isolate individual thermal degradation products. As can be seen, the intensity of the detected species varies dramatically as a function of the polymer composition. Ions that correlate to all of the important features of the total ion profiles are easily identified. This greatly simplifies the interpretation of the data and distinguishes preferable regions of the spectrum for background subtraction. This is illustrated in Figure 7 wherein the small peak at 0.65 min (339 amu) from Figure 3B has been isolated and simplified through subtraction of the signal on either side of the peak. Such data processing techniques are necessary for the interpretation of multi-component electron impact mass spectrum.
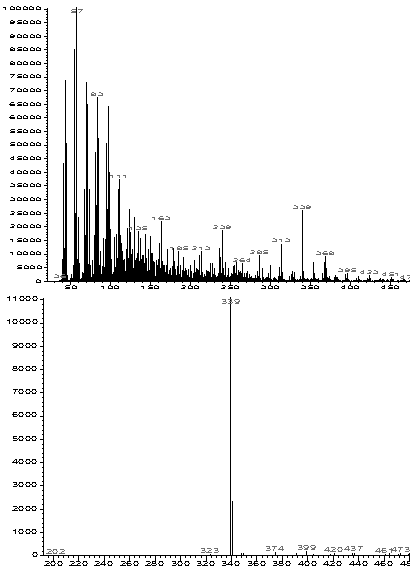
Figure 7. Background Subtraction of a Small Component Identified Through Extraction Ion Signal. A. Raw Mass Spectral Data. B. Background Subtracted.
Conclusions
We have demonstrated that data indicating the relative composition of Ethylene/Vinyl Acetate copolymers can be obtained quickly using a direct probe and the HP 5973 MSD. The data manipulation capabilities of the HP ChemStation software are required to interpret multi-component electron impact mass spectra. The results indicate that simplified quality control procedures should be possible using the above techniques. Further work will be done to examine the sensitivity of this approach for the detection of impurities.


