- ▶
- Heaters/Source
- ▶
- Agilent Heaters and SensorsMass Spectrometry, Scientific Supplies & ManufacturingScientific Instrument Services 5973 Source Heater Tamper Resistant Allen Wrench 5973/5975 Quad Sensor 5985 Source Heater Assembly Agilent Interface Heater Assembly 5971 Interface Heater

- ▶
- Reference Material on InstrumentationArticle - A High Temperature Direct Probe for a Mass Spectrometer Design of a Direct Exposure Probe and Controller for use ona Hewlett-Packard 5989 Mass Spectrometer SIS AP1000 AutoProbe™ SIS AP2000 AutoProbe™ - Description of System HPP7: Direct Probe Electronics Console HPP7: Direct Probe for the Agilent (HP) 5973/5975 MSD HPP7: HP Direct Probe Application Notes HPP7: Installation Directions for the Direct Probe HPP7: Side Cover for the HP 5973 MSD HPP7: Support HPP7: Probe Inlet System for the Agilent (HP) 5973 and 5975 MSD with Automatic Indexed Stops HPP7: Theory of Operation of the Direct Probe and Probe Inlet System Direct Thermal Extraction Thermal Desorption Application Notes Environmental Thermal Desorption Application Notes Food Science Thermal Desorption Application Notes Forensic Thermal Desorption Application Notes GC Cryo-Trap Application Notes Headspace Application Notes Purge & Trap Thermal Desorption Application Notes Theory of Operation of the AutoDesorb® System AutoDesorb Notes for SIS Dealers Adsorbent Resin Application Notes Installation of the Short Path Thermal Desorption System on Agilent (HP) and Other GCs Installation of the Short Path Thermal Desorption System on a Varian 3400 GC AutoDesorb® System Development Team Thermal Desorption Applications and Reference Materials Installation of the Short Path Thermal Desorption System - TD5 Part I - Design & Operation of the Short Path ThermalDesorption System Installation Instructions for the Model 951 GC Cryo-Trap on the HP 5890 Series GC Installation Instructions for the Model 961 GC Cryo-Trap on the HP 5890 Series GC Operation of the Model 951/961 GC Cryo-Trap SIS GC Cryo Traps - Theory of Operation NIST/EPA/NIH Mass Spectral Enhancements - 1998 version (NIST98) SIMION 3D Ion Optics Class Mass Spectrometer Source Cleaning Methods MS Tip: Mass Spectrometer Source Cleaning Procedures Mass Spec Source Cleaning Procedures Micro-Mesh® Abrasive Sheets Research Papers Using New Era Syringe Pump Systems EI Positive Ion Spectra for Perfluorokerosene (PFK) Cap Liner Information How do I convert between fluid oz and milliliters? Which bottle material should I choose? Which bottle mouth should I choose? The Bottle Selection Guide CGA Connections for Gas Tanks Chemical Reaction Interface Mass Spectrometry (CRIMS)

- TD
- ▶
- AccessoriesTD Supply Kit Desorption Tubes Adsorbent Resins Desorption Tube Needles Desorption Tube Seals Desorption System Fittings GC Cryo-Trap Extraction Cell TD Sample Loader Prepacked, Conditioned Desorption Tubes Desorption Tube Packing Accessories Stainless Steel Purge Heads Injection Port Liners Tenax TA Poster TD Application Notes Customer Service

- LiteratureApplication Notes Adsorbent Resins Guide Mass Spec Tips SDS Sheets FAQ MS Calibration Compound Spectra Manuals MS Links/Labs/ Organizations MS Online Tools Flyers on Products/Services Scientific Supplies Catalog About Us NextAdvance Bullet Blender® Homogenizer Protocols Micro-Mesh® Literature Instrumentation Literature Agilent GC/MS Literature SIS News / E-Mail Newsletter NIST MS Database - Update Notifications

- ▶
- Thermal Desorption Applications and Reference MaterialsDirect Thermal Extraction Headspace Environmental Food Science Applications Pharmaceuticals Forensic Note 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 57: Aroma Profiles of Lavandula species Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS

- Application NotesNote 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 96: Reducing Warping in Mass Spectrometer Filaments, with SISAlloy® Yttria/Rhenium Filaments Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 92: Yttria Coated Mass Spectrometer Filaments Note 91: AutoProbe DEP Probe Tip Temperatures Note 90: An Automated MS Direct Probe for use in an Open Access Environment Note 89: Quantitation of Organics via a Mass Spectrometer Automated Direct Probe Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 87: Design and Development of an Automated Direct Probe for a Mass Spectrometer Note 86: Simulation of a Unique Cylindrical Quadrupole Mass Analyzer Using SIMION 7.0. Note 85: Replacing an Electron Multiplier in the Agilent (HP) 5973 MSD Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 81: Rapid Bacterial Chemotaxonomy By DirectProbe/MSD Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 78: A New Solution to Eliminate MS Down-Time With No-Tool-Changing of Analytical GC Columns Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 76: Determination of the Sensitivity of a CRIMS System Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 72: 1998 Version of the NIST/EPA/NIH Mass Spectral Library, NIST98 Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 70: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part II Note 69: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part 1 Note 68: Use of a PC Plug-In UV-Vis Spectrometer To Monitor the Plasma Conditions In GC-CRIMS Note 67: Using Chemical Reaction Interface Mass Spectrometry (CRIMS) To Monitor Bacterial Transport In In Situ Bioremediation Note 66: Probe Tip Design For the Optimization of Direct Insertion Probe Performance Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization Note 61: Analysis of Sugars Via a New DEP Probe Tip For Use With theDirect Probe On the HP5973 MSD Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 59: Computer Modeling of a TOF Reflectron With Gridless Reflector Using SIMION 3D Note 58: Direct Probe Analysis and Identification of Multicomponent Pharmaceutical Samples via Electron Impact MS Note 57: Aroma Profiles of Lavandula species Note 56: Mass Spec Maintenance & Cleaning Utilizing Micro-Mesh® Abrasive Sheets Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 53: SIMION 3D v6.0 Ion Optics Simulation Software Note 52: Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry Using SIMION 3D Note 51: Development and Characterization of a New Chemical Reaction Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) Note 50: The Analysis of Multiple Component Drug Samples Using a Direct Probe Interfaced to the HP 5973 MSD Note 49: Analysis of Cocaine Utilizing a New Direct Insertion Probe on a Hewlett Packard 5973 MSD Note 48: Demonstration of Sensitivity Levels For the Detection of Caffeine Using a New Direct Probe and Inlet for the HP 5973 MSD Note 47: The Application Of SIMION 6.0 To Problems In Time-of-Flight Mass Spectrometry Note 46: Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond Note 45: Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources Note 44: The Design Of a New Direct Probe Inlet For a Mass Spectrometer Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS Tech No. "A" Note 14: Elimination of "Memory" Peaks in Thermal Desorption Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers - Part I of II Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers- Part II of II Adsorbent Resins Guide Development and Field Tests of an Automated Pyrolysis Insert for Gas Chromatography. Hydrocarbon Production in Pine by Direct Thermal Extraction A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Capillary (019P) - Comparison of Septa by Direct Thermal Extraction Volatile Organic Composition in Blueberry Identification of Volatile Organic Compounds in Office Products Detection and Indentification of Volatiles in Oil Base Paintsby Headspace GC with On Column Cryo-Trapping Evaluation of Septa Using a Direct Thermal Extraction Technique INFLUENCE OF STORAGE ON BLUEBERRY VOLATILES Selection of Thermal Desorption and Cryo-Trap Parameters in the Analysis of Teas Redesign and Performance of a Diffusion Based Solvent Removal Interface for LC/MS The Design of a New Direct Probe Inlet for a Mass Spectrometer Analytes Using Mass Spectrometry (CRIMS) Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources A Student Guide for SIMION Modeling Software Application of SIMION 6.0 to Problems in Time-of-flight Mass Spectrometry Comparison of Sensitivity of Headspace GC, Purge and TrapThermal Desorption and Direct Thermal Extraction Techniques forVolatile Organics The Influence of Pump Oil Purity on Roughing Pumps Analysis of Motor Oils Using Thermal Desorption-Gas Chromatography-Mass Spectrometry IDENTIFICATION OF VOLATILE ORGANIC COMPOUNDS IN PAPER PRODUCTS Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry using SIMION 3D Seasonal Variation in Flower Volatiles Development of and Automated Microprocessor Controlled Gas chromatograph Fraction Collector / Olfactometer Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Column Design of a Microprocessor Controlled Short Path Thermal Desorption Autosampler Computer Modeling of Ion Optics in Time-of-Flight Mass Spectrometry Using SIMION 3D Thermal Desorption Instrumentation for Characterization of Odors and Flavors

- ▶
- Note 57: Aroma Profiles of Lavandula species (This Page)
By Eleanor Wiesenfeld, Noville, South Hackensack, NJ
12/23/99

Abstract
The procedure for determining aroma profiles of various species of live Lavandula plants is discussed. Techniques employed include: dynamic headspace purge, short-path thermal desorption, GC/MSD/FID.
INTRODUCTION
The Lavandula genus consists of about 20 species of small evergreen shrubs having aromatic foliage and flowers (1). The genus is a member of the Labiatae family, along with the thymes, mints, rosemary, sage, and other herbs, sharing with them the characteristic squared stems, two-lipped flowers and paired leaves (2). The plants are useful as decorative hedges in the garden, while the dried flowers are used in potpourris, as cooking herbs (3), and as insect repellents (7). Several species grow wild in the rocky soil of Southern Europe: Spain, Portugal, Southern France, etc. In the past, these wild plants were harvested by the local people and distilled for their essential oil (9). At present, fields are cultivated for this purpose in the calcareous soil of Mediterranean countries as well as other areas, especially Bulgaria and the countries of the former Yugoslavia (5).
Commercially, the Lavandula genus provides several important essential oils to the fragrance industry. Because of this, the composition of these oils has been extensively investigated (4). However, little has been written concerning the actual aroma of the various lavender species, that is, the aroma profile as determined by headspace analysis.
The aroma profile is determined by analyzing the headspace of a growing plant, a non-destructive technique. In comparison, the essential oil is produced by steam distillation of the above-ground parts (flowers, leaves and stems) of harvested lavender plants (5). Due to the harsh conditions prevalent during the distillation process, one would expect the odor profile of the living plant to be different than that of the processed oil. The aroma profile may be thought of as correlating to the perceived aroma of the living plant. The oil, however, reflects the composition of volatiles and semi-volatiles present in the plant, with molecular transformations occurring during the distillation process and during storage. Adulteration with synthetic chemicals (principally, linalool and linalyl acetate) and other essential oils of lesser value, is also a common practice. In this paper, aroma profiles of various Lavandula species will be investigated. In addition, commercially available lavender oil will be compared to its respective aroma profile.
Experimental
Botanicals
One-year-old Lavandula plants were obtained from a supplier and identified as to species. Upon arrival, the plants were repotted and acclimated in the laboratory for several weeks. The specimens were sampled after each put forth active growth. All sampling took place within a three month period, prior to flowering. Each specimen was sampled at least twice, and the results averaged. The lavender species involved in this study were: L. officinalis or angustifolia (English Lavender), L. dentata (French or fringed Lavender), L. stoechas (Spanish Lavender), L. spica, L. viridis (green lavender), L. lanata (wooly lavender) (11), L. pinnata, L. multifida, and L. x heterophylla 'Goodwin Creek'. Of these, L. angustifolia is of commercial interest as the source of an essential oil used extensively in the fragrance industry.
Nomenclature of the individual species is sometimes confusing and contradictory and the common names are sometimes inconsistent. For instance, Lavandula angustifolia, usually referred to as English lavender, grows wild in Southern France. Lavandula stoechas, which grows wild in Spain, is sometimes referred to as French Lavender. Additionally, Lavandula spica is thought to be a non-specific name, possibly a form of angustifolia (8). To eliminate confusion, common names will not be referred to in the text.
Sampling and Analysis Technique
In preparation for sampling, several stems of each plant are enclosed in a two-piece spherical, custom-designed glass vessel, taking care to not damage or stress the plant. The two sections of the vessel are clamped around the stems and, over the course of several hours, the headspace is sampled by means of a low-flow vacuum pump. The volatile components are trapped on an adsorbent resin (Tenax® or equivalent), which is packed within a desorption tube attached to the pump. Subsequently, these trapped components are desorbed by means of a short path thermal desorption unit(10) into a gas chromatograph equipped with a cryo trap (10). A blank is purged and analyzed to determine artifacts from the system.
Instruments and conditions: The GC/MS (Hewlett Packard 5890/5971) is configured with two matched (50 meter, methyl silicone) capillary columns, one inlet, and two detectors (MSD and FID). The inlet is modified to accept the thermal desorption unit. The cryo trap is installed at the head of the columns and cooled to -50oC by carbon dioxide. Desorption takes place at 220oC for 4 minutes. After desorption is completed, the cryo trap is heated to match the inlet temperature, while the GC temperature is programmed from 350C to 2400C at 3oC per minute.
Data Analysis
A proprietary, mass spectrum library provides peak identification. Quantitation
is determined by FID area normalization. Because of the complexity of the
chromatograms, no correction is made for response differences.
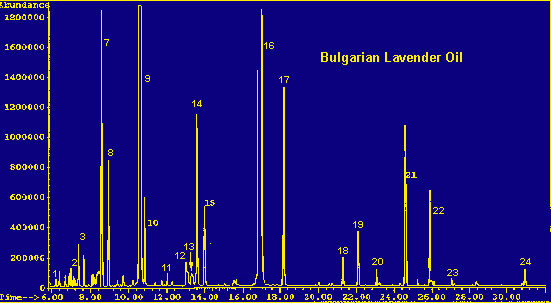
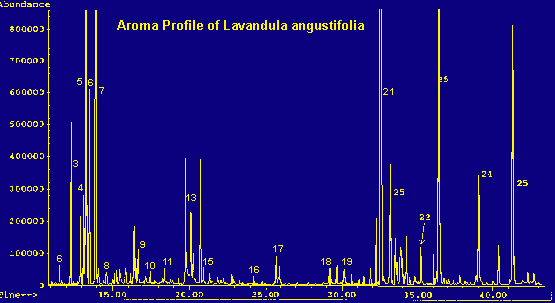
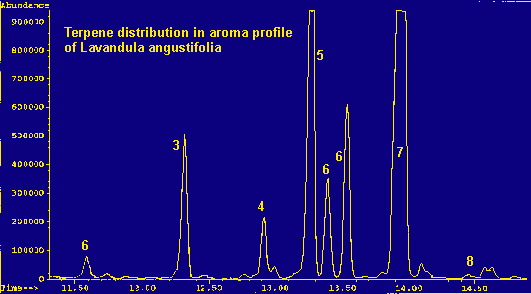
Legend For Chromatographs
Number Component
- alpha-thujene
- sabinene
- myrcene
- alpha-phellandrene
- delta-3-carene
- cymenes (3 isomers)
- multi component peak*
- trans-ocimene
- linalool
- octenyl acetate
- camphor
- lavandulol
- borneol
- terpinene-4-ol
- alpha-terpineol
- linalyl acetate
- lavnandulyl acetate
- neryl acetate
- geranyl acetate
- coumarin
- caryophyllene
- farnesene
- germacrene
- caryophyllene oxide
- other sesquiterpenes
* may include: beta-phellandrene, cis-ocimene, limonene, and 1,8-cineoleResults
All results are reported as averages; refer to Tables 1 and 2 for the
data. Although no ranges are given in the results, in some instances the
variation is considerable. In spite of attempts to standardize the sampling
parameters, differences still occur due to the inherent variable nature
of living things. However, despite the quantitative differences, in general,
the peak pattern of each specimen's profile is maintained from one sampling
to another.
| Table 1 - AROMA PROFILES
OF SELECTED LAVANDULA SPECIES
All data semi-quantitative--results expressed as percent Species with 'typical' scent |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| * sum of isomers
** may include beta-phellandrene, cis-ocimene, limonene, cineole--see text |
| Table 2 - AROMA PROFILES
OF SELECTED LAVANDULA SPECIES
All data semi-quantitative--results expressed as percent Species with 'a typical' scent |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| * sum of isomers
** may include beta-phellandrene, cis-ocimene, limonene, cineol |
Terpenes
Because of the large number of components present in each of the samples and the nature of the non-polar column, coelution is practically inevitable. This occurs in the case of beta-phellandrene, limonene, cis-ocimene, and 1,8-cineole (a terpene oxide). An exact quantitation of their distribution within the peak cannot be determined, but an approximation can be made by mass spectrometry. (See tables 1 and 2 for totals.) An analysis of this peak shows: limonene dominates in multifida, pinnata, and heterophylla; cineole dominates in viridis, dentata, lanata, and spica. Lavandula angustifolia contains a greater percentage of beta-phellandrene and L. stoechas seems to contain almost equal amounts of limonene and cineole.
Cymene most commonly occurs as the para isomer. However, Lavandula angustifolia shows two other isomers of cymene in addition to the para form. Presumably, these are meta and ortho, but this has not been proven. Quantitatively, all the cymenes are added together in tables 1 and 2.
Sesquiterpenes
The sesquiterpenes are reported as a sum of the various isomers (beta, gamma, delta, etc.) for that particular sesquiterpene. Those sesquiterpenes listed under 'other sesquiterpenes' are mostly unknown, but are usually not the same unknown.
Diterpenes
The tentative identification of unknown diterpenes found in L. viridis, L. stoechas, and L. lanata is made by molecular weight, as determined by mass spectrometry molecular ion.
Discussion
In the following discussion the scent of each Lavandula species will be correlated with the composition of its respective aroma profile derived by dynamic headspace analysis.
The Lavenders
These Lavandulas can be grouped by chemotype, that is chemically, according to the major components in their aroma profiles. Some are dominated by terpenes and terpene oxides (viridis, angustifolia, dentata, and spica), others fall into the high camphor classification (stoechas, lanata), while still others are in the ocimene/carvacrol group (pinnata and multifida). The scent of most, but not all Lavandulas, is somewhat similar namely: refreshing, herbaceous and sweet, imparting a sense of 'clean'. In addition to having a similar scent, most lavenders have a similar appearance as well: small shrubs with narrow, flat, gray-green leaves (7). But, there are always exceptions. Lavandula dentata has the characteristic scent, but a rather distinctive leaf, being bright green and finely toothed (7). Lavandula species pinnata and multifida have a distinctly different scent and appearance than the others in this study. They are warm and balsamic, while their appearance is rather like a fern, having finely cut grayish leaves. Unlike the other lavenders, L. x heterophylla "Goodwin Creek" has a flat, greenish-gray, tomentose leaf. It exhibits a rather atypical aroma, being more floral and less herbaceous than the others.
Terpenes
Coelution of the four terpenes and terpene oxide in the 'multi-component peak' (see tables) is significant due to the abundance of this peak in the chromatograms and the differing olfactive properties of the various components. The aroma of the plant can depend upon which component dominates this complex peak. Beta-phellandrene is 'peppery-minty and slightly citrusy', contributing to the herbaceous scent of the angustifolia species. Cineole is 'camphoraceous, cool', adding a substantial sharp, penetrating quality to the viridis, dentata, lanata and spica species. Limonene is either dextro or laevo: d-limonene being 'sweet citrusy', whereas l-limonene is 'very clean, not reminiscent of Citrus fruits'. Limonene dominates the terpene fraction of the heterophylla species and contributes to the scent of stoechas. Trans-ocimene, the dominant terpene in the pinnata and multifida species, has a "warm-herbaceous odor", enhancing the balsamic qualities of the carvacrol component (all descriptions see ref. 6).
Sesquiterpenes
The total sesquiterpene content of the different species varies notably, from 1.7% in L. stoechas to 35.9% in L. angustifolia. Referring to tables 1 and 2, the variety of sesquiterpenes is distinctive for each species, and this unique distribution undoubtedly contributes to the variation in scent. The high amount of caryophyllene, along with a considerable amount of cadinenes, in L. angustifolia contributes to the distinctive woody note found in this species. It is not surprising to see a large amount of bisabolenes (sweet-spicy-balsamic (6)) in the pinnata and multifida species, enhancing their warm, sweet, spicy scent. Positive identification of individual sesquiterpenes can be difficult, if not impossible. All have the same molecular formula (C15H24) and although the molecular configuration of each is different, the fragmentation pattern can sometimes be remarkably similar, thus making mass spec identification imprecise. Retention time can sometimes be used in determining differences, but not to confirm identification. In some cases, retention time data was used to declare a sesquiterpene 'unknown'.
Lavandulol/Borneol
Lavandulol and borneol cannot always be separated by the non-polar column used for this study. However, these two alcohols can be differentiated by mass spectrometry. The two species containing lavandulol in the effluent are L. x heterophylla and L. lanata. Of these, heterophylla contains a significant amount of this terpene alcohol and its scent reflects this. Lavandulol, an isomer of geraniol, has a 'warm-rosy odor', 'with a slightly spicy note' (6). This, along with the floralcy of the germacrenes, likely contributes to the uncharacteristically floral note in the heterophylla specimen.
Borneol, more widely distributed throughout the genus than lavandulol, has a 'dry-camphoraceous, woody-peppery odor' (6), more characteristic of the lavenders. This terpene alcohol, along with its acetate and ketone form (camphor), adds to the distinctive warm, minty, herbaceous aroma of the typical lavender.
Comparison of Aroma Profile To Distilled Oil
Since these aroma profiles are produced by headspace analysis of the
leaves and stems of living plants, while the essential oils are prepared
by distillation of the leaves, stems, and flowers of harvested plants,
comparisons between the oil and headspace compositions are of limited value.
We can assume that inclusion of the flowers in the oil greatly influences
its composition. However, since comparisons will inevitably be made, one
is included here.
| Table 3 - Composition
of
Lavandula angustifolia aroma profile compared to Bulgarian Lavender Oil (results expressed as percent) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| * may include beta-phellandrene, cis-ocimene,
limonene, cineole
** contains approximately 30% of a tricyclo sesquiterpene Aroma profile is from dynamic headspace purge Bulgarian Oil is steam distilled |
Table 3 compares the composition of the aroma profile of Lavandula angustifolia with that of the steam distilled oil of Bulgarian lavender, presumably derived mostly from this same species. (Hortus Third gives the source of Lavender Oil as Lavandula angustifolia and Lavandula stoechas (8). This may refer to the plants growing wild in Southern France and Spain as harvested in years past. Most other sources give L. angustifolia, the cultivated species, as the usual source for the oil. It can be assumed that this commercially available oil is derived from cultivated plants.)
Lavender oil of Bulgarian origin was chosen for this comparison because:
1) a large part of the lavender oil commercially available today is of Bulgarian origin;
2) the sample used for analysis was considered reliably free of adulterants; and
3) the composition of the sample is typical for a lavender oil.
Referring to the table, the most obvious differences between the headspace and the distilled oil are in the amounts of linalool and linalyl acetate. The level of linalyl acetate is of great interest in a lavender oil, because the quality of the oil is evaluated by its ester content; the higher the ester content, the finer the oil. Therefore, the low percentage of these two components in the headspace of the leaves is of particular interest. Again, exclusion of the flowers from the aroma profile, is an important point and may account for most of the disparity. However, the inherent differences between a headspace sampling and a steam distillation method cannot be discounted as a factor. Due to the high temperatures and harsh conditions prevailing during the distillation process, this technique often leads to degradation products and molecular rearrangements. Storage time of the oil must also be considered as a factor.
Another interesting difference between the composition of the headspace and the oil is the distribution of the sesquiterpenes. Both contain caryophyllene, a sesquiterpene found widely in nature. However, the headspace contains approximately four percent of a tricyclo sesquiterpene eluting on the back of the caryophyllene peak. This peak does not appear in the oil. In addition, the oil contains farnesene and germacrene isomers, while the headspace contains considerable amounts of cadinene and bergamotene isomers (listed as 'other sesquiterpenes' in the legend).
In general, the headspace is dominated by terpenes and sesquiterpenes, while the oil contains mostly alcohols and esters.
Summary
Dynamic headspace analysis coupled with thermal desorption is a powerful technique for studying the correlation of effluent composition with scent in living plants. A reliable mass spectrometry library is a necessity for identification of the components.
This work was not intended to be a exhaustive study of the subject, but the data can be used for comparative purposes and as a guide to direct future projects.
Future studies will involve analyzing the flowers of various Lavandula species, especially those of L. angustifolia to determine their contribution to the oil composition.
Acknowledgment
The author wishes to thank Sandra Seals and Laura Ferrara for their help in preparing this presentation.
Presented at Pitt Con 97, Atlanta, GA, March 1997.
References
(1) Allaby, M., (editor), The Concise Oxford Dictionary of Botany, Oxford University Press, 1992.
(2) Van Pelt Wilson, H., and Bell, L., The Fragrant Year, M. Barrows & Company, Inc., 1967.
(3) Janulewics, M., Traditional Home Book of Herbs, Longmeadow Press, 1996.
(4) Boelens, Perfumer and Flavorist, May/June 1995.
(5) The H&R Book of Perfume.
(6) Arctander, S., Perfume and Flavor Chemicals, vols. 1&2, Published by the author, original publication 1961.
(7) Bremner, Lesly, Complete Book of Herbs, Penguin Books, 1994.
(8) Hortus Third, Macmillan, 1976.
(9) Arctander, Steffen, Perfume and Flavor Materials of Natural Origin, Published by the author, original publication 1961.
(10) Available from Scientific Instruments Services, Ringoes, NJ
(11) Brown, Deni, Encylopedia of Herbs, and Their Uses, Dorling Kindersley, 1995.

