- ▶
- Heaters/Source
- ▶
- Agilent Heaters and SensorsMass Spectrometry, Scientific Supplies & ManufacturingScientific Instrument Services 5973 Source Heater Tamper Resistant Allen Wrench 5973/5975 Quad Sensor 5985 Source Heater Assembly Agilent Interface Heater Assembly 5971 Interface Heater

- ▶
- Reference Material on InstrumentationArticle - A High Temperature Direct Probe for a Mass Spectrometer Design of a Direct Exposure Probe and Controller for use ona Hewlett-Packard 5989 Mass Spectrometer SIS AP1000 AutoProbe™ SIS AP2000 AutoProbe™ - Description of System HPP7: Direct Probe Electronics Console HPP7: Direct Probe for the Agilent (HP) 5973/5975 MSD HPP7: HP Direct Probe Application Notes HPP7: Installation Directions for the Direct Probe HPP7: Side Cover for the HP 5973 MSD HPP7: Support HPP7: Probe Inlet System for the Agilent (HP) 5973 and 5975 MSD with Automatic Indexed Stops HPP7: Theory of Operation of the Direct Probe and Probe Inlet System Direct Thermal Extraction Thermal Desorption Application Notes Environmental Thermal Desorption Application Notes Food Science Thermal Desorption Application Notes Forensic Thermal Desorption Application Notes GC Cryo-Trap Application Notes Headspace Application Notes Purge & Trap Thermal Desorption Application Notes Theory of Operation of the AutoDesorb® System AutoDesorb Notes for SIS Dealers Adsorbent Resin Application Notes Installation of the Short Path Thermal Desorption System on Agilent (HP) and Other GCs Installation of the Short Path Thermal Desorption System on a Varian 3400 GC AutoDesorb® System Development Team Thermal Desorption Applications and Reference Materials Installation of the Short Path Thermal Desorption System - TD5 Part I - Design & Operation of the Short Path ThermalDesorption System Installation Instructions for the Model 951 GC Cryo-Trap on the HP 5890 Series GC Installation Instructions for the Model 961 GC Cryo-Trap on the HP 5890 Series GC Operation of the Model 951/961 GC Cryo-Trap SIS GC Cryo Traps - Theory of Operation NIST/EPA/NIH Mass Spectral Enhancements - 1998 version (NIST98) SIMION 3D Ion Optics Class Mass Spectrometer Source Cleaning Methods MS Tip: Mass Spectrometer Source Cleaning Procedures Mass Spec Source Cleaning Procedures Micro-Mesh® Abrasive Sheets Research Papers Using New Era Syringe Pump Systems EI Positive Ion Spectra for Perfluorokerosene (PFK) Cap Liner Information How do I convert between fluid oz and milliliters? Which bottle material should I choose? Which bottle mouth should I choose? The Bottle Selection Guide CGA Connections for Gas Tanks Chemical Reaction Interface Mass Spectrometry (CRIMS)

- TD
- ▶
- AccessoriesTD Supply Kit Desorption Tubes Adsorbent Resins Desorption Tube Needles Desorption Tube Seals Desorption System Fittings GC Cryo-Trap Extraction Cell TD Sample Loader Prepacked, Conditioned Desorption Tubes Desorption Tube Packing Accessories Stainless Steel Purge Heads Injection Port Liners Tenax TA Poster TD Application Notes Customer Service

- LiteratureApplication Notes Adsorbent Resins Guide Mass Spec Tips SDS Sheets FAQ MS Calibration Compound Spectra Manuals MS Links/Labs/ Organizations MS Online Tools Flyers on Products/Services Scientific Supplies Catalog About Us NextAdvance Bullet Blender® Homogenizer Protocols Micro-Mesh® Literature Instrumentation Literature Agilent GC/MS Literature SIS News / E-Mail Newsletter NIST MS Database - Update Notifications

- ▶
- Thermal Desorption Applications and Reference MaterialsDirect Thermal Extraction Headspace Environmental Food Science Applications Pharmaceuticals Forensic Note 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 57: Aroma Profiles of Lavandula species Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS

- ▶
- Food Science Thermal Desorption Application NotesNote 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 43: Volatile Organic Composition In Blueberries Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 35: Volatile Organics Composition of Cranberries Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 25: Flavor and Aroma in Natural Bee Honey Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS

- Environmental Thermal Desorption Application NotesNote 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing

- Purge & Trap Thermal Desorption Application NotesNote 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing

- Application NotesNote 103: EPA Method 325B, Novel Thermal Desorption Instrument Modification to Improve Sensitivity Note 102: Identification of Contaminants in Powdered Beverages by Direct Extraction Thermal Desorption GC/MS Note 101: Identification of Contaminants in Powdered Foods by Direct Extraction Thermal Desorption GC/MS Note 100: Volatile and Semi-Volatile Profile Comparison of Whole Versus Cracked Versus Dry Homogenized Barley Grains by Direct Thermal Extraction Note 99: Volatile and Semi-Volatile Profile Comparison of Whole vs. Dry Homogenized Wheat, Rye and Barley Grains by Direct Thermal Extraction GC/MS Note 98: Flavor and Aroma Profiles of Truffle Oils by Thermal Desorption GC/MS Note 97: Flavor Profiles of Imported and Domestic Beers by Purge & Trap Thermal Desorption GC/MS Note 96: Reducing Warping in Mass Spectrometer Filaments, with SISAlloy® Yttria/Rhenium Filaments Note 95: Detection of Explosives on Clothing Material by Direct and AirSampling Thermal Desorption GC/MS Note 94: Detection of Nepetalactone in the Nepeta Cataria Plant by Thermal Desorption GC/MS Note 93: Detection of Benzene in Carbonated Beverages with Purge & Trap Thermal Desorption GC/MS Note 92: Yttria Coated Mass Spectrometer Filaments Note 91: AutoProbe DEP Probe Tip Temperatures Note 90: An Automated MS Direct Probe for use in an Open Access Environment Note 89: Quantitation of Organics via a Mass Spectrometer Automated Direct Probe Note 88: Analysis of Silicone Contaminants on Electronic Components by Thermal Desorption GC-MS Note 87: Design and Development of an Automated Direct Probe for a Mass Spectrometer Note 86: Simulation of a Unique Cylindrical Quadrupole Mass Analyzer Using SIMION 7.0. Note 85: Replacing an Electron Multiplier in the Agilent (HP) 5973 MSD Note 84: Vacuum Pump Exhaust Filters - Charcoal Exhaust Traps Note 83: Vacuum Pump Exhaust Filters - Oil Mist Eliminators Note 82: Vacuum Pump Exhaust Filters Note 81: Rapid Bacterial Chemotaxonomy By DirectProbe/MSD Note 80: Design, Development and Testing of a Microprocessor ControlledAutomated Short Path Thermal Desorption Apparatus Note 79: Volatile Organic Compounds From Electron Beam Cured and Partially Electron Beam Cured Packaging Using Automated Short Path Thermal Desorption Note 78: A New Solution to Eliminate MS Down-Time With No-Tool-Changing of Analytical GC Columns Note 77: The Determination of Volatile Organic Compounds in VacuumSystem Components Note 76: Determination of the Sensitivity of a CRIMS System Note 75: An Apparatus for Sampling Volatile Organics From LivePlant Material Using Short Path Thermal Desorption Note 74: Examination of Source Design in Electrospray-TOF Using SIMION 3D Note 73: The Analysis of Perfumes and their Effect on Indoor Air Pollution Note 72: 1998 Version of the NIST/EPA/NIH Mass Spectral Library, NIST98 Note 71: Flavor Profile Determination of Rice Samples Using Shor tPath Thermal Desorption GC Methods Note 70: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part II Note 69: Application of SIMION 6.0 To a Study of the Finkelstein Ion Source: Part 1 Note 68: Use of a PC Plug-In UV-Vis Spectrometer To Monitor the Plasma Conditions In GC-CRIMS Note 67: Using Chemical Reaction Interface Mass Spectrometry (CRIMS) To Monitor Bacterial Transport In In Situ Bioremediation Note 66: Probe Tip Design For the Optimization of Direct Insertion Probe Performance Note 65: Determination of Ethylene by Adsorbent Trapping and Thermal Desorption - Gas Chromatography Note 64: Comparison of Various GC/MS Techniques For the Analysis of Black Pepper (Piper Nigrum) Note 63: Determination of Volatile and Semi-Volatile Organics in Printer Toners Using Thermal Desorption GC Techniques Note 62: Analysis of Polymer Samples Using a Direct Insertion Probe and EI Ionization Note 61: Analysis of Sugars Via a New DEP Probe Tip For Use With theDirect Probe On the HP5973 MSD Note 60: Programmable Temperature Ramping of Samples Analyzed ViaDirect Thermal Extraction GC/MS Note 59: Computer Modeling of a TOF Reflectron With Gridless Reflector Using SIMION 3D Note 58: Direct Probe Analysis and Identification of Multicomponent Pharmaceutical Samples via Electron Impact MS Note 57: Aroma Profiles of Lavandula species Note 56: Mass Spec Maintenance & Cleaning Utilizing Micro-Mesh® Abrasive Sheets Note 55: Seasonal Variation in Flower Volatiles Note 54: Identification of Volatile Organic Compounds in Office Products Note 53: SIMION 3D v6.0 Ion Optics Simulation Software Note 52: Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry Using SIMION 3D Note 51: Development and Characterization of a New Chemical Reaction Interface for the Detection of Nonradioisotopically Labeled Analytes Using Mass Spectrometry (CRIMS) Note 50: The Analysis of Multiple Component Drug Samples Using a Direct Probe Interfaced to the HP 5973 MSD Note 49: Analysis of Cocaine Utilizing a New Direct Insertion Probe on a Hewlett Packard 5973 MSD Note 48: Demonstration of Sensitivity Levels For the Detection of Caffeine Using a New Direct Probe and Inlet for the HP 5973 MSD Note 47: The Application Of SIMION 6.0 To Problems In Time-of-Flight Mass Spectrometry Note 46: Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond Note 45: Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources Note 44: The Design Of a New Direct Probe Inlet For a Mass Spectrometer Note 43: Volatile Organic Composition In Blueberries Note 42: The Influence of Pump Oil Purity on Roughing Pumps Note 41: Hydrocarbon Production in Pine by Direct Thermal Extraction Note 40: Comparison of Septa by Direct Thermal Extraction Note 39: Comparison of Sensitivity Of Headspace GC, Purge and Trap Thermal Desorption and Direct Thermal Extraction Techniques For Volatile Organics Note 38: A New Micro Cryo-Trap For Trapping Of Volatiles At the Front Of a GC Capillary Column Note 37: Volatile Organic Emissions from Automobile Tires Note 36: Identification Of Volatile Organic Compounds In a New Automobile Note 35: Volatile Organics Composition of Cranberries Note 34: Selection Of Thermal Desorption and Cryo-Trap Parameters In the Analysis Of Teas Note 33: Changes in Volatile Organic Composition in Milk Over Time Note 32: Selection and Use of Adsorbent Resins for Purge and Trap Thermal Desorption Applications Note 31: Volatile Organic Composition in Several Cultivars of Peaches Note 30: Comparison Of Cooking Oils By Direct Thermal Extraction and Purge and Trap GC/MS Note 29: Analysis Of Volatile Organics In Oil Base Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 28: Analysis Of Volatile Organics In Latex Paints By Automated Headspace Sampling and GC Cryo-Focusing Note 27: Analysis of Volatile Organics In Soils By Automated Headspace GC Note 26: Volatile Organics Present in Recycled Air Aboard a Commercial Airliner Note 25: Flavor and Aroma in Natural Bee Honey Note 24: Selection of GC Guard Columns For Use With the GC Cryo-Trap Note 23: Frangrance Qualities in Colognes Note 22: Comparison Of Volatile Compounds In Latex Paints Note 21: Detection and Identification Of Volatile and Semi-Volatile Organics In Synthetic Polymers Used In Food and Pharmaceutical Packaging Note 20: Using Direct Thermal Desorption to Assess the Potential Pool of Styrene and 4-Phenylcyclohexene In Latex-Backed Carpets Note 19: A New Programmable Cryo-Cooling/Heating Trap for the Cryo-Focusing of Volatiles and Semi-Volatiles at the Head of GC Capillary Columns Note 18: Determination of Volatile Organic Compounds In Mushrooms Note 17: Identification of Volatile Organics in Wines Over Time Note 16: Analysis of Indoor Air and Sources of Indoor Air Contamination by Thermal Desorption Note 14: Identification of Volatiles and Semi-Volatiles In Carbonated Colas Note 13: Identification and Quantification of Semi-Volatiles In Soil Using Direct Thermal Desorption Note 12: Identification of the Volatile and Semi-Volatile Organics In Chewing Gums By Direct Thermal Desorption Note 11: Flavor/Fragrance Profiles of Instant and Ground Coffees By Short Path Thermal Desorption Note 10: Quantification of Naphthalene In a Contaminated Pharmaceutical Product By Short Path Thermal Desorption Note 9: Methodologies For the Quantification Of Purge and Trap Thermal Desorption and Direct Thermal Desorption Analyses Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System Note 7: Chemical Residue Analysis of Pharmaceuticals Using The Short Path Thermal Desorption System Note 6: Direct Thermal Analysis of Plastic Food Wraps Using the Short Path Thermal Desorption System Note 5: Direct Thermal Analysis Using the Short Path Thermal Desorption System Note 4: Direct Analysis of Spices and Coffee Note 3: Indoor Air Pollution Note 2: Detection of Arson Accelerants Using Dynamic Headspace with Tenax® Cartridges Thermal Desorption and Cryofocusing Note 1: Determination of Off-Odors and Other Volatile Organics In Food Packaging Films By Direct Thermal Analysis-GC-MS Tech No. "A" Note 14: Elimination of "Memory" Peaks in Thermal Desorption Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers - Part I of II Improving Sensitivity in the H.P. 5971 MSD and Other Mass Spectrometers- Part II of II Adsorbent Resins Guide Development and Field Tests of an Automated Pyrolysis Insert for Gas Chromatography. Hydrocarbon Production in Pine by Direct Thermal Extraction A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Capillary (019P) - Comparison of Septa by Direct Thermal Extraction Volatile Organic Composition in Blueberry Identification of Volatile Organic Compounds in Office Products Detection and Indentification of Volatiles in Oil Base Paintsby Headspace GC with On Column Cryo-Trapping Evaluation of Septa Using a Direct Thermal Extraction Technique INFLUENCE OF STORAGE ON BLUEBERRY VOLATILES Selection of Thermal Desorption and Cryo-Trap Parameters in the Analysis of Teas Redesign and Performance of a Diffusion Based Solvent Removal Interface for LC/MS The Design of a New Direct Probe Inlet for a Mass Spectrometer Analytes Using Mass Spectrometry (CRIMS) Application of SIMION 6.0 to Filament Design for Mass Spectrometer Ionization Sources A Student Guide for SIMION Modeling Software Application of SIMION 6.0 to Problems in Time-of-flight Mass Spectrometry Comparison of Sensitivity of Headspace GC, Purge and TrapThermal Desorption and Direct Thermal Extraction Techniques forVolatile Organics The Influence of Pump Oil Purity on Roughing Pumps Analysis of Motor Oils Using Thermal Desorption-Gas Chromatography-Mass Spectrometry IDENTIFICATION OF VOLATILE ORGANIC COMPOUNDS IN PAPER PRODUCTS Computer Modeling of Ion Optics in Time-of-Flight mass Spectrometry using SIMION 3D Seasonal Variation in Flower Volatiles Development of and Automated Microprocessor Controlled Gas chromatograph Fraction Collector / Olfactometer Delayed Extraction and Laser Desorption: Time-lag Focusing and Beyond A New Micro Cryo-Trap for the Trapping of Volatiles at the Front of a GC Column Design of a Microprocessor Controlled Short Path Thermal Desorption Autosampler Computer Modeling of Ion Optics in Time-of-Flight Mass Spectrometry Using SIMION 3D Thermal Desorption Instrumentation for Characterization of Odors and Flavors

- ▶
- Note 8: Detection of Volatile Organic Compounds In Liquids Utilizing the Short Path Thermal Desorption System (This Page)
INTRODUCTION
Short Path Thermal Desorption is a versatile technique for the analyses of solid, liquid and gaseous samples. Previous articles in this newsletter have described the theory and operation of short path thermal desorption (1) and have demonstrated its versatility in the analysis of volatile organic chemicals (VOC's) and semi-volatile organic chemicals in solid matrices (2 & 3). The detection and analysis of VOC's in water is routinely performed utilizing Purge and Trap techniques with standard EPA methodology (4). The detection of VOC's in other water-based matrices presents its own unique problems. The purpose of this article is to demonstrate the versatility of the short path thermal desorption technique to the analyses of VOC's in commercial water based products including liquid formulations, colloidal suspensions, and liquid pastes. Using a newly designed liquid purging system for the collection of the VOC's followed by trapping on an adsorbent trap and subsequent analysis via the Short Path Thermal Desorption technique, it is possible to detect and identify the various flavors, fragrances, off-flavors, off-odors, and manufacturing by-products in this wide diversity of liquid samples. This technique can easily be incorporated into a troubleshooting technique to detect problems in a wide variety of liquid products, to compare various competing manufacturers products, as well as a quality control program.
Volatile organic compounds are present in commercial liquid formulations, a diverse range of colloidal suspensions, and liquid pastes. Flavors and fragrances are added for their aesthetic values and to increase consumer appeal. The flavor/fragrance qualities of liquid commercial products is greatly dependent on the plethora of volatile and semivolatile organic compounds contained both in the liquid matrix and the headspace aroma (5). Volatile compounds are also used in the manufacturing process to obtain the desired physical properties. Trace residues of these manufacturing by-products are often present in the final products. Analytical techniques are needed to profile and identify flavors, fragrances, off-flavors, off-odors and potential contaminants that may be present as flavor and fragrance additives, residual solvents from the manufacturing process or as impurities in raw materials. Results of such analyses may be used for developmental research as well as production quality control.
The detection of VOC's from liquids can be accomplished by using a headspace sampling technique combined with gas chromatography (GC) and/or gas chromatography/mass spectrometry (GC/MS). The most sensitive of the techniques is the dynamic headspace technique. Dynamic headspace analysis depends on the vapor phase extraction of the sample liquid achieved by purging it with an inert gas. VOC's present will diffuse into the vapor phase and are collected on a solid sorbent material, as Tenax®. Older desorption type purge and trap methodology and apparatus, such as utilized by the EPA, is only applicable to very volatile components, as solvents.
However, the Short Path Thermal Desorption system permits the analyses of liquid samples by desorbing the samples previously collected on adsorbent resins directly into the GC injection port for subsequent analyses by conventional GC detectors or via mass spectrometers. Due to its Short path of sample flow, this new system overcomes the shortcomings of previous desorption systems by eliminating transfer lines which are easily contaminated by samples and by providing for the optimum delivery and therefore maximum sensitivity of samples to the GC injector via the shortest path possible (1). By making the transfer path as short as possible, the maximum sample size is delivered to the GC, samples are not lost or destroyed in hot transfer lines, and no memory effects occur due to contamination of transfer lines from previous samples. The high recovery rates, time savings for sample preparation and the high sensitivity of short path thermal desorption make this not only an attractive technique to the analyst but an essential detection apparatus because of toxicology and quality control, as well as a means for determining human health impacts.
The objective of this investigation is to demonstrate the feasibility of combining a headspace sampling technique with the technique of Short Path Thermal desorption to analyze VOC's that can be purged from diverse liquids, colloidal suspensions, or water-based pastes. Commercial beverages including fruit drinks, carbonated colas, and wine coolers as well as olive oil, shampoo, latex paint, toothpaste and a spiked water sample were analyzed by thermal desorption-gas chromatography-mass spectrometry (TD-GC-MS) using a Scientific Instrument Services (S.I.S.) model TD-1 Short Path Thermal Desorber accessory.
Instrumentation
A. Liquid Purging System
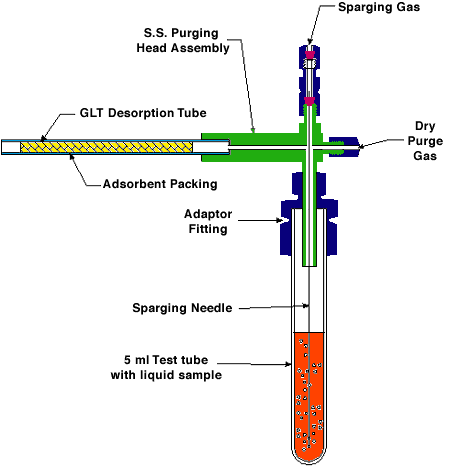
Figure 1a. Schematic of Enhanced Dry Purge Headspace Sampling Apparatus With 5 ml Test Tube
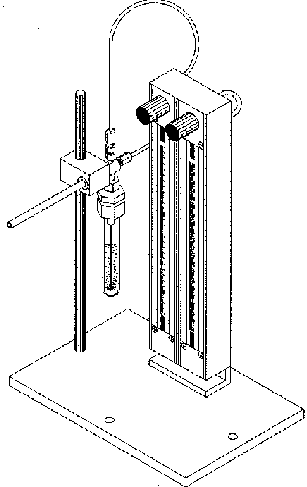
Figure 1b. Liquid Purging System
Samples were collected using a S.I.S. enhanced dry purge headspace sampling apparatus. This apparatus (Fig. 1a) consists of a sparge gas inlet connected to stainless steel purging needle that is inserted through an adaptor fitting into a 50 ml round bottom flask or into a 5 ml test tube (Figs. 1a & b). The dry purge gas inlet is located at a right angle to the sparge gas inlet at the top of the apparatus (Figs. 1a & b). This can be left in the closed or open position. The purpose of the dry purge is to reduce the water vapor condensation on the adsorbent trap. This problem can be especially troublesome when isolating volatiles from aqueous solutions at high temperatures. Although the adsorbent traps packed with Tenax have a low affinity for water, it is inevitable that some condensation will occur in the trap due to the high relative humidity of the sparge gas as it exits the apparatus. When moisture condenses on the adsorbent, it can block the pores of the resin matrix and thereby drastically reduce the diffusion of volatile organics into the trapping agents. This will result in reduced trapping efficiency. Opposite the dry purge inlet is the connector for the glass-lined stainless steel (GLT) desorption tube (Fig 1b). Many different sizes of glassware can be used as the collection vessel for the sample depending on the size of the sample to be analyzed.
The liquid purging system contains two ball rotameters with adjustable needle valves mounted on a stationary base and permit the visual indication and independent adjustment of the carrier gas flow to each of the gas inlets (Fig. 1b). The 150 mm long flow tube contains a sapphire ball for flow ranges of 0 to 72 ml/min of helium or air.
B. GC, GC/MS
Initial screening of samples was performed on a Varian 3400 GC with a flame ionization detector in order to determine the sample size and thermal desorption temperature and time. Split ratios of 1 to 20 were used for overloaded samples. Otherwise, direct splitless analysis was used. The GC column was a 30 meter x .32 mm i.d. DB-5 capillary column containing a 1.0 um film thickness with a flow rate of 2.0 ml per min (He). The column was temperature programmed from -40 degrees C (hold for 10 minutes to cryofocus during thermal desorption interval) to 280 degrees C at a rate of 10 degrees C per minute.
Sample identification was performed on an HP 5890 GC interfaced to an HP 5971 MSD. The GC injector was maintained at 260 degrees C. The GC column was a 25 meter x .25 mm i.d. DB-5 capillary column containing a 0.25 um film thickness with a flow rate of 1.0 ml per minute (He). The column was temperature programmed from -40 degrees C (hold for 10 minutes to cryofocus during thermal desorption interval) to 280 degrees C at a rate of 10 degrees C per minute.
C. Short Path Thermal Desorption System
All experiments were conducted using a S.I.S. model TD-1 Short Path Thermal Desorber accessory connected either to a Varian 3400 gas chromatograph or an HP 5890 GC interfaced to an HP 5971 MSD. The theory and operation of the Short Path Thermal Desorption System was previously described in this newsletter (1). GLT desorption tubes were purged for 10 minutes at 40 ml/min to remove any excess water and then thermally desorbed for 10 minutes at a temperature of 150 degrees C to 200 degrees C.
Samples to be analyzed are collected on silanized glass-lined stainless steel desorption tubes packed with 100 mg. of Tenax TA 60/80 mesh. The ends of the tubes are plugged with silanized glass wool approximately one centimeter on each end. Using an S.I.S. Desorption Tube Conditioning System, traps are then temperature programmed from ambient temperature to 320 degrees C at a rate of 4 degrees C per minute while purging with helium at a flow rate not less than 20 ml per minute. The traps are held at the upper temperature limit for not less than four hours. After conditioning, the tubes are promptly sealed on both ends using column end caps fitted with PTFE ferrules. Traps prepared in this manner exhibit excellent adsorptive capacity and contain no organic background (bleed or artificial peaks) when analyzed by GC-MS.
Experimental
Sample sizes of 1 to 25 mls of various commercial products were measured in a graduated cylinder and transferred to a 5 ml test tube or a 50 ml round bottom flask. Samples were sparged with helium at 15 to 30 ml/min with an additional 15 to 30 ml/min dry purge for 10 to 15 minutes using a S.I.S. enhanced dry purge headspace sampling apparatus. All samples were collected at room temperature. Volatile analytes were gas extracted and carried to a preconditioned 4.0 mm i.d. glass-lined stainless steel desorption tube packed with 100 mg of Tenax TA. The tubes were then fitted with S.I.S. syringe adaptors and connected to the TD-1 Thermal Desorber.
Results and Discussion
The following liquid based products depict the wide diversity of commercial liquid products that can be analyzed via this technique. All the samples were analyzed by purging as described above at room temperature. Additional compounds of less volatility could have been detected if the samples were heated, and this may be desirable in many instances. Liquid samples with low concentrations of foreign materials such as the spiked water sample are the easiest type of sample to analyze. No foaming occurred and the volatiles are easily purged from these samples. The commercial beverages contain many more compounds and begin to foam. In many instances, it is necessary to use larger vessels, foam breakers, or other techniques to minimize these foam forming problems. Colloidal solutions, as the latex paint and shampoo, present additional problems due to their thick nature. It may be preferable to dilute these samples with additional water to make them less viscous and enhance the purging of the VOC's. Liquid pastes, as toothpaste, are the final extreme of sample density and need to be diluted in order to purge the volatiles. In addition, foaming is a major problem and large vessels (relative to the sample size) are required to prevent the foam from entering the desorption trap.
Water Samples
A calibration mixture (Polyscience) containing 2 mg/ml in methanol of benzene, toluene, 2-bromo-1-chloropropane (int. std.), m-xylene, p-isopropyltoluene and naphthalene was diluted in glass distilled water with a final concentration of 20 ppm. Figure 2 shows a chromatogram of the components as they are separated by gas chromatography. A desorption temperature of 200 degrees C proved to be the optimum temperature for the detection of the less volatile compound naphthalene.
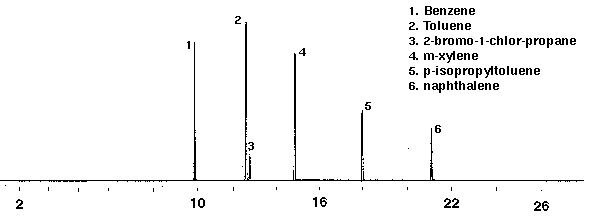
Figure 2 - Gas Chromatograph of Calibration Mixture, 50 ml. Collected For 15 min at 20 ml/m with 20 ml/m Dry Purge Thermally Desorbed at 200 Degrees C for 10 min.
Fruit Drinks
Several brands of non-alcoholic fruit drinks were studied to identify the flavor volatiles present. The fruit drinks were found to contain numerous mono-and sesquiterpenoid compounds and flavors such as ethyl acetate, ethyl butyrate and benzaldehyde (Figs. 3, 4 & 5). The negative control (empty desorption tube) was found to be free of interferences with the exception of two minor peaks which were organosilicone compounds from the injection port system bleed. Monoterpenes were identified as limonene in orange, cranberry and raspberry drinks (Figs. 3, 4 & 5) and terpinene and myrcene in orange (Fig. 3). Sesquiterpenes included cadinene in orange (Fig. 3) and caryophyllene in raspberry (Fig. 5). The Merck Index lists these compounds as constituents of several plant derived essential oils which are used as fragrance materials.
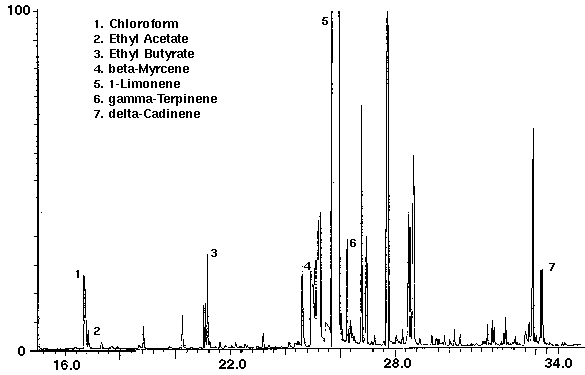
Figure 3 - Orange drink, 25 ml. Collected For 10 min at 30 ml/m With 30 ml/m Dry Purge Thermally Desorbed At 150 Degrees C For 10 min.
The flavor ethyl acetate was detected in all drinks with ethyl butyrate found in orange and cranapple (Figs. 3 & 4). High concentrations of the flavors furfural and 1-butanol, -3-methyl-, acetate were found in cranberry (Fig. 4) and raspberry (Fig. 5), respectively. A trace amount of chloroform was identified in orange (Fig. 3) as well as trace amounts of methyl chloroform in cranapple and raspberry (Figs. 4 & 5). The aromatic compounds toluene and xylene were also detected in raspberry (Fig. 5). Trace amounts of these compounds may be present as residual solvents from the manufacturing process or occur naturally. Styrene which may have leached from the plastic cap liner was found in cranapple (Fig. 4).
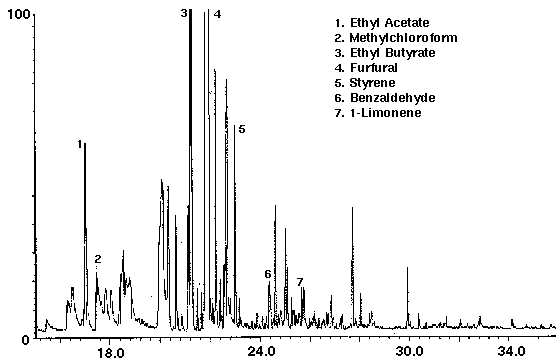
Figure 4 - Cranapple drink, 25 ml. Collected For 10 min at 30 ml/m With 30 ml/m Dry Purge Thermally Desorbed At 150 Degrees C For 10 min.
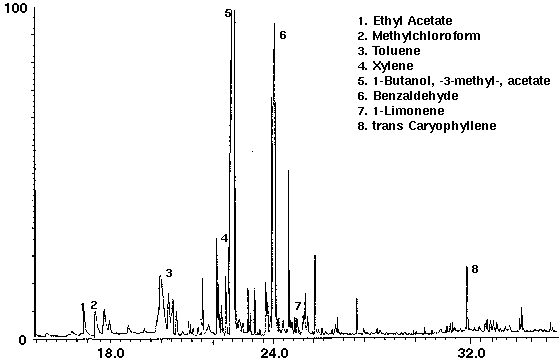
Figure 5 - Orange drink, 25 ml. Collected For 10 min at 30 ml/m With 30 ml/m Dry Purge Thermally Desorbed At 150 Degrees C For 10 min.
Carbonated Colas
Several brands of carbonated colas were analyzed to determine the feasibility of this technique to distinguish the different manufacturers brands. Monoterpenes such as camphene, cymene, limonene and terpinene were identified in both of the carbonated colas, in addition to the alcohols endo-fenchol and endo-borneol (Figs. 6 & 7). While high concentrations of cymene and limonene were detected in Cola A (Fig. 6), Cola B was found to contain high concentrations of isocineole and 1,8 cineole (Fig. 7). Trace amounts of chloroform, most likely from water treatment, were found to be present in each of the colas. Cola A was also found to contain the aromatic compound toluene (Fig. 6) which may be present as a natural product from essential oils, synthetic or as a residue from the manufacturing process.
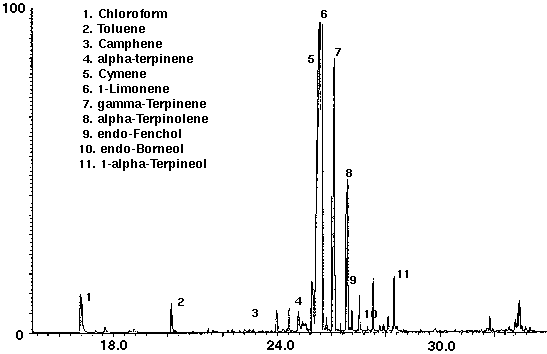
Figure 6 - Cola A, 25 ml. Collected for 10 min At 15 ml/m With 10 ml/m Dry Purge Thermally Desorbed At 150 Degrees C For 10 min.
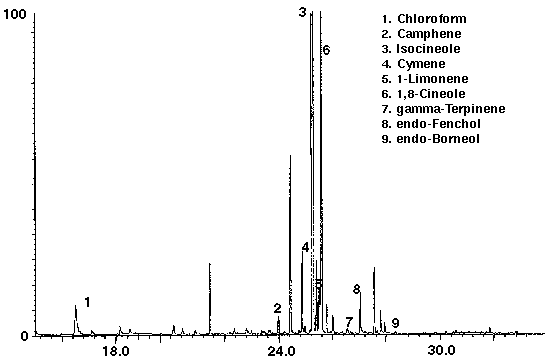
Figure 7 - Cola B, 25 ml. Collected For 10 min At 15 ml/m With 10 ml/m Dry Purge Thermally Desorbed At 150 Degrees C For 10 min.
Wine Coolers
Alcohol containing wine coolers were analyzed to demonstrate the variation of the flavor compounds in these drinks. The dry purge step in the sample set-up purges the ethanol from the sample in addition to water. Even though each wine cooler had its own distinct chromatograph, they were found to contain common compounds such as the flavors ethyl acetate, ethyl butyrate, ethyl caproate and propyl acetate, as well as several monoterpenes with limonene the most common (Figs. 8 & 9). Additional compounds which were found in the wine coolers included aldehyde and alcohol derivatives.
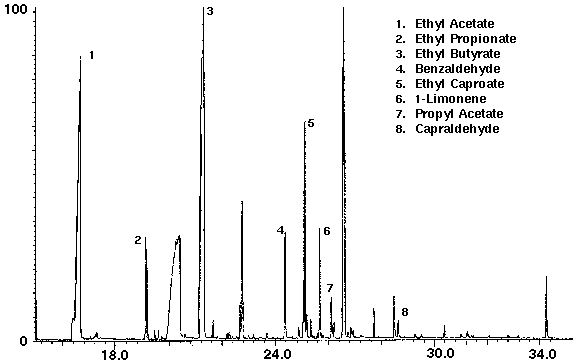
Figure 8 - Wine Cooler A, 25 ml. Collected For 10 min at 30 ml/m With 30 ml/m Dry Purge 20:1 split Thermally Desorbed At 150 Degrees C For 10 min.
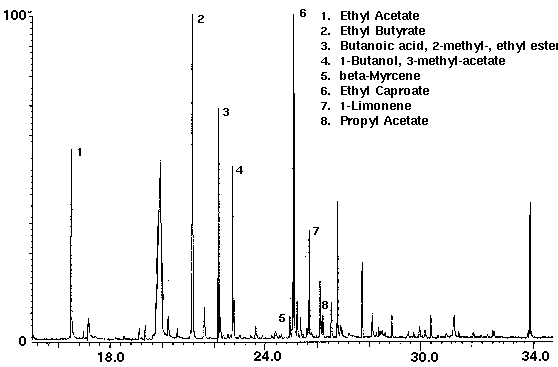
Figure 9 - Wine Cooler B, 25 ml. Collected For 10 min At 30 ml/m With 30 ml/m Dry Purge 20:1 Split Thermally Desorbed At 150 Degrees C For 10 min.
Olive Oil
The analysis of olive oil demonstrates the detection of volatiles from other non-water based liquids. A series of straight chain hydrocarbons and linear alkenes such as 1- and 2-octene from lipid oxidation decomposition products were identified in the olive oil sample (Fig. 10). Minor constituents, as ketones, aldehydes and alcohols, were identified, as well as trace amounts of the aromatic compounds benzene and toluene (Fig. 10). The presence of these compounds may be the result of the manufacturing process or occur naturally from essential essences. Olive oil was also found to contain the compound styrene.
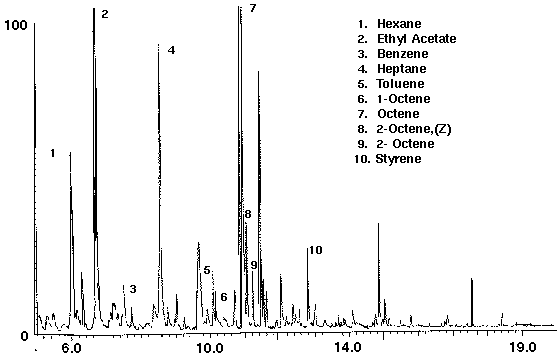
Figure 10 - Olive Oil, 5 ml. Collected For 10 min at 20 ml/m With 20 ml/m Dry Purge 20:1 Split Thermally Desorbed At 150 Degrees C For 10 min.
Shampoo
A popular hair shampoo was analyzed to determine the volatiles which could be purged from a thick water based suspension. Shampoo was found to contain numerous monoterpenoid compounds such as alpha and beta pinene, camphene, terpinene, linalool and a high concentration of limonene (Fig. 11). Several ketones, in addition to a trace amount of the solvent 1,4-dioxane for removing oils from hair, were identified. A high concentration of the fragrance compound benzyl acetate which occurs in a number of plants, particularly jasmine, and linalyl acetate, a valuable constituent of bergamot and lavender oils, were also detected (Fig. 11). Additional compounds included a series of sesquiterpenoid compounds such as alpha-copaene and caryophyllene.
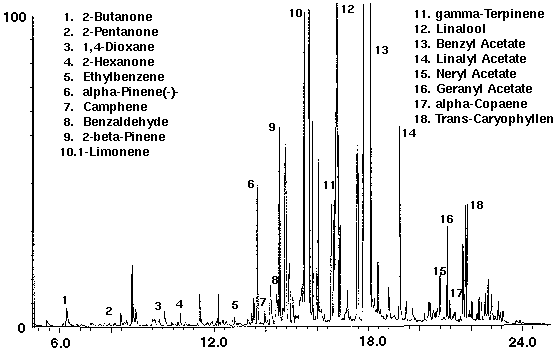
Figure 11 - Shampoo, 5 ml. Collected For 10 min at 20 ml/m With 20 ml/m Dry Purge 20:1 Split Thermally Desorbed At 150 Degrees C For 10 min.
Latex Paint
The identification and quantitation of VOC's in latex paints is of importance to paint manufacturers. A latex enamel paint was found to contain over 100 volatile organic compounds (Fig. 12). Numerous straight and branched chain hydrocarbons and alcohols were identified. Butyl esters of propanoic and butanoic acids were also found in latex enamel paint (Fig. 13). In addition to a number of ketone compounds, trace amounts of the aromatic compounds benzene, toluene and other benzene derivatives were present (Fig. 13).
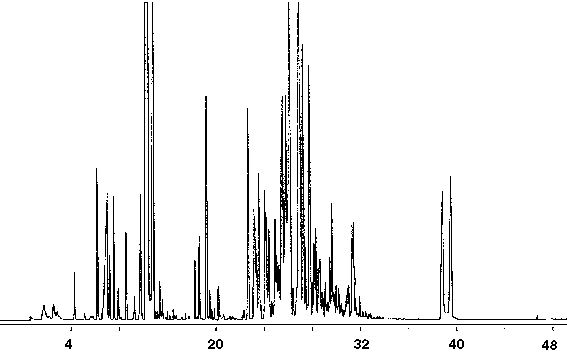
Figure 12 - Gas Chromatograph of Latex Enamel Paint, 1 ml Collected For 10 min at 15 ml/m With 15 ml/m Dry Purge Thermally Desorbed At 150 Degrees C For 10 min
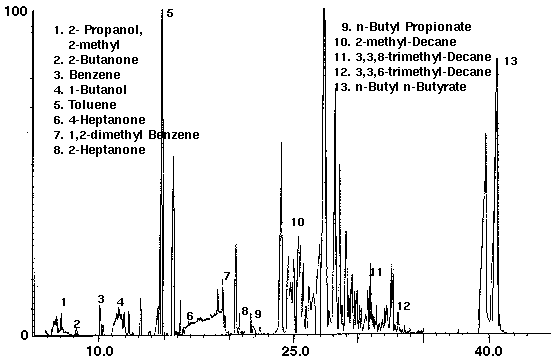
Figure 13 - Latex Enamel Paint, 1 ml Collected For 10 min at 15 ml/m With 15 ml/m Dry Purge Thermally Desorbed At 150 Degrees C For 10 min
Toothpaste
A method is needed to detect the volatile manufacturing by products in toothpaste. Liquid paste, as toothpaste, was diluted 1:5 in glass distilled water to enhance the purging of VOC'S. The collection time was reduced to 6 minutes due to sample foaming; however, the liquid paste was found to contain over 50 VOC'S (Fig. 14). The sample foams precautions must be taken to prevent the foam from reaching and contaminating the adsorbent trap. This can be accomplished using a foam breaker such as a distillation trap which is usually used on rotary evaporators. It is fitted directly below the adsorbent trap. As an alternative, a larger collection vessel or a tube packed with a small amount of glass wool often suffices to break the foam and allow the liquid to drop back down into the vessel. A heated dynamic headspace purging technique may also be used where helium or another suitable gas is purged over the surface of a heated sample.
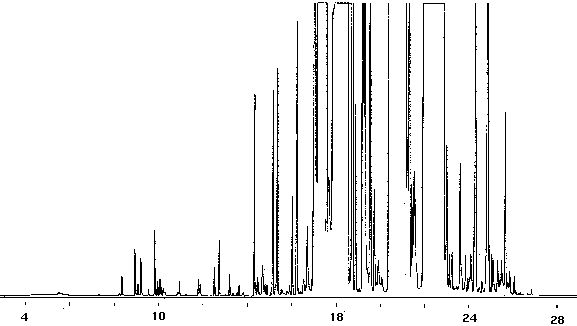
Figure 14 - Gas Chromatography of Toothpaste Diluted In Glass-Distilled Water (1.5), 5 ml Collected For 6 min at 20 ml/m With 20 ml/m Dry Purge Thermally Desorbed At 150 Degrees C For 10 min
Conclusion
The S.I.S. model TD-1 Short Path Thermal Desorber accessory, used in combination with GC-MS is an ideal instrument for the detection of volatile organics from a liquid matrix. It can quickly and easily analyze a small amount of sample to yield both qualitative and quantitative data on volatile organic composition. In this study, the apparatus was successfully employed for the determination of volatile components in a variety of liquid matrices. Combined with the dynamic headspace sampling technique, the technique of Short Path Thermal Desorption can be used during the production of commercial products, for quality control of flavor/fragrance additives as well as for the detection of residual solvents.
References
Food Science Applications. Food Technology. Vol. 45 (7): 104-105.
EPA Methods for the Determination of Organic Compounds in Drinking Water. 1988. Environmental Monitoring Systems Laboratory-Cincinnati. EPA-600/4-88/039. 378 pp.
Hartman, T. G., J. Lech and R. T. Rosen. 1990. Determination of Off-Odors and Other Volatile Organics in Food Packaging Films by Direct Thermal Analysis-GC-MS. The Mass Spec Source. Vol. XIII (4): 30-33.
Hartman, T. G., S. V. Overton, J. J. Manura, C. W. Baker and J. N. Manos. 1991. Short Path Thermal Desorption:
Manura, J. J. 1991. Direct Thermal Analysis Using the Short Path Thermal Desorption System. The Mass Spec Source. Vol. XIV (1): 22-27.
Manura, J. J., S. V. Overton, C. W. Baker and J. N. Manos. 1990. Short Path Thermal Desorption-Design and Theory. The Mass SpecSource. Vol. XIII (4): 22-28.

